BenzePrO Foam Prescribing Information
Package insert / product label
Generic name: benzoyl peroxide
Dosage form: aerosol, foam
Drug class: Topical acne agents
Medically reviewed by Drugs.com. Last updated on Feb 28, 2024.
On This Page
Indications and Usage for BenzePrO Foam
Indicated for the topical treatment of mild to moderate acne vulgaris
Warnings
For external use only
When using this product
- avoid unnecessary sun exposure and use a sunscreen
- avoid contact with eyes, lips, and mouth
- avoid contact with hair and dyed fabrics, which may be bleached by this product
- skin irritation may occur, characterized by redness, burning, itching, peeling, possibly swelling. Irritation may be reduced by using the product less frequently or in a lower concentration
BenzePrO Foam Dosage and Administration
See package insert for full prescribing information
Prime can before initial use: See package insert Before Each Use: Shake vigorously
During Use: Holding can upright, dispense into palm of hand and apply to affected area as directed by physician.
- If going outside, apply sunscreen after using this product. If irritation or sensitivity develops, stop use of both products and ask a doctor.
Other Information
- Store at room temperature 15°-25° C (59°-77° F)
- Protect from freezing
- Store upright
Inactive Ingredients
BHT, C12-15 alkyl benzoate, cetostearyl alcohol, citric acid, dimethicone, disodium EDTA, emulsifying wax, glycerin, methylparaben, povidone, propylene glycol, propylparaben, purified water, sodium citrate, steareth-10, stearic acid, trolamine. Also contains: Propellant HFA-134A (1, 1, 1, 2-tetrafluoroethane).
Questions? 866-696-8525
Manufactured for:
PruGen, Inc.
Pharmaceuticals
18899 North Thompson Peak Parkway
Scottsdale, Arizona 85255 REV 1.2
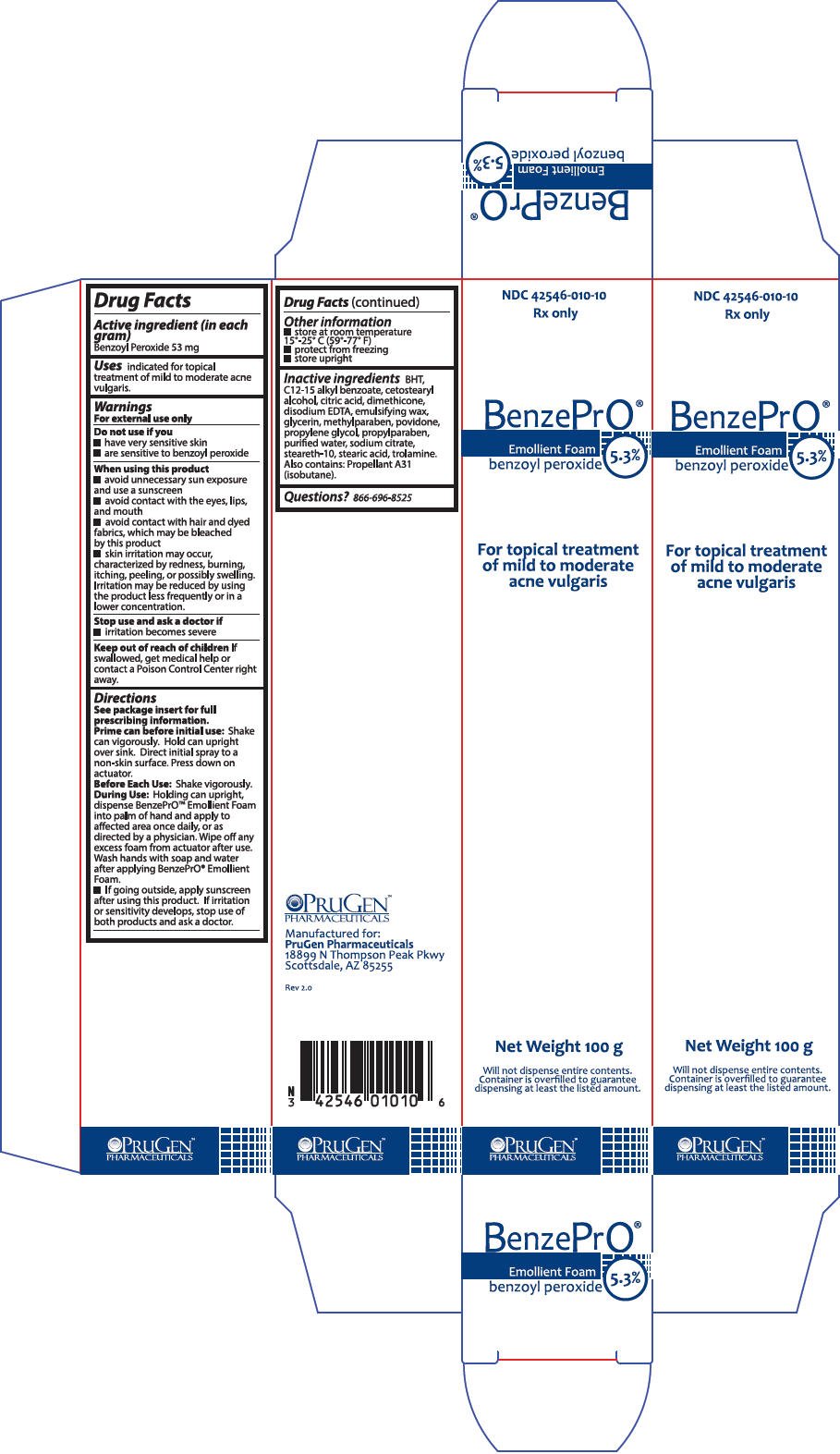
PRINCIPAL DISPLAY PANEL - 100 g Can Box
NDC 42546-010-10
Rx only
BenzePrO®
Emollient Foam
benzoyl peroxide
5.3%
For topical treatment
of mild to moderate
acne vulgaris
Net Weight 100 g
Will not dispense entire contents.
Container is overfilled to guarantee
dispensing at least the listed amount.
PRUGEN™
PHARMACEUTICALS
| BENZEPRO
benzoyl peroxide aerosol, foam |
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
| Labeler - PruGen, Inc. (929922750) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| PHARMASOL CORPORATION | 065144289 | MANUFACTURE(42546-010) | |
Frequently asked questions
More about BenzePro (benzoyl peroxide topical)
- Check interactions
- Compare alternatives
- Side effects
- Dosage information
- During pregnancy
- Drug class: topical acne agents
- Breastfeeding
- En español
Patient resources
Professional resources
- BenzePrO Creamy Wash prescribing information
- BenzePrO Foaming Cloths (FDA)
- BenzePrO Short Contact Foam (FDA)
Other brands
Epsolay, BPO 6 Foaming Cloths, Enzoclear Foam
