Vistaseal Dosage
Generic name: Fibrinogen Human 80mg in 1mL; Thrombin Human 500[iU] in 1mL
Dosage form: injection
Drug class: Miscellaneous coagulation modifiers
Medically reviewed by Drugs.com. Last updated on Mar 24, 2023.
For topical use only.
Dosage
Individualize application of the fibrin sealant. Individual doses typically ranged from 0.3 to 18.0 mL in the clinical studies. Larger volumes may be required for surgical procedures other than those included in the clinical studies.
The approximate surface area coverage for each VISTASEAL package size is provided in Table 1.
| VISTASEAL package size | Surface area coverage (cm2) Application by dripping or spray (1 mm thick layer) |
| 2 mL | 16 - 22 |
| 4 mL | 32 - 44 |
| 6 mL | 48 - 66 |
| 10 mL | 80 - 110 |
Dose depends on variables including, but not limited to, the type of surgical intervention, the size of the area, the intended application method, and the number of applications.
Apply a sufficient volume of VISTASEAL to entirely cover the intended application area with a thin layer. Repeat the application if necessary.
Preparation and Handling
Prepare and administer the product only according to the instructions and with the recommended devices.
Remove carton from freezer, open it and take out the two blisters.
Place the blister containing the VISTASEAL Dual Applicator at room temperature until the VISTASEAL Fibrin Sealant (Human) is ready to use.
Room Temperature Thawing (preferred method)
Thaw blister with VISTASEAL pre-filled syringes at room temperature using the following steps:
- Place the blister containing the syringe holder with pre-filled syringes on a surface at room temperature (20 ºC - 25 ºC, [68 - 77 ºF])
for approximately 70 minutes for the 2 mL and the 4 mL package sizes
for approximately 90 minutes for the 6 mL and the 10 mL package sizes
After thawing, it is not necessary to warm the product for its use.
After thawing, the solutions must be clear to slightly opalescent and colorless to pale yellow.
Do not use solutions that are cloudy or have deposits.
Post-Thawing Storage:
After thawing, the kit containing the VISTASEAL syringe holder with pre-filled syringes and Dual Applicator can be stored before use for not more than 48 hours in the refrigerator at 2 ‑ 8 ºC [36 - 46 ºF] or 24 hours at room temperature (20 - 25 °C [68 - 77 ºF]) if it remains sealed in the original packaging. Once the blisters are opened, use VISTASEAL immediately during the surgery and discard any unused contents.
Once thawed, do not refreeze.
Transferring instructions:
- After thawing, remove the blister from the surface at room temperature or from the refrigerator at 2 - 8 °C [36 - 46 ºF].
- Open the blister and make the VISTASEAL syringe holder with pre-filled syringes available to a second person for transfer to the sterile field. The outside of the blister should not come in contact with the sterile field. See Figure 1.
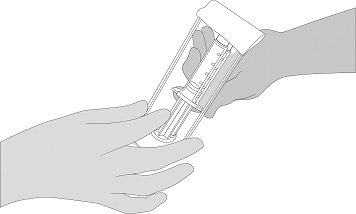
Figure 1
Sterile Water Bath (Quick Thawing)
Thaw VISTASEAL pre-filled syringes inside the sterile field in a sterile thermostatic water bath at a temperature not higher than 37 ºC [99 ºF] using the following steps:
NOTE: Once the VISTASEAL blisters are opened, use the product immediately during surgery. Use sterile technique to avoid the possibility of contamination due to improper handling, and follow the steps below accurately. Do not remove the syringe luer cap until thawing is complete and the Dual Applicator is ready to be attached.
- Open the blister and make the VISTASEAL syringe holder with pre-filled syringes available to a second person for transfer to the sterile field. The outside of the blister should not come in contact with the sterile field. See Figure 1.
- Place the syringe holder with pre-filled syringes directly into the sterile water bath ensuring that it is completely immersed in the water. See Figure 2.
- At 37 ºC, the time needed is approximately 5 minutes for the 2 mL, 4 mL, 6 mL, and 10 mL package sizes, but must not be left at this temperature for longer than 10 minutes.
The temperature of the water bath must not exceed 37 ºC. - Dry the syringe holder with pre-filled syringes after thawing, using a sterile surgical gauze.
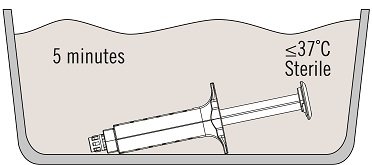
Figure 2
After thawing, the solutions must be clear to slightly opalescent and colorless to pale yellow.
Do not use solutions that are cloudy or have deposits.
Use VISTASEAL immediately during the surgery and discard any unused contents.
Connection instructions
- Open the blister and make the VISTASEAL Dual Applicator and two additional Airless Spray Tips available to a second person for transfer to the sterile field. The outside of the blister should not come in contact with the sterile field.
- Hold the VISTASEAL Fibrin Sealant (Human) syringe holder with syringe luer caps pointed upward. See Figure 3.
- Unscrew and discard the syringe luer cap of both fibrinogen and thrombin syringes. See Figure 3.
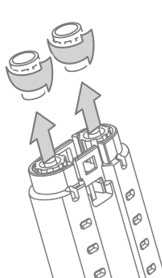
Figure 3
- Hold the syringe holder with the luers pointed upward. To remove air bubbles from syringes, strike gently the side of the syringe holder one or two times while keeping the syringe holder in an upright position and lightly depress the plunger to eject air. See Figure 4.
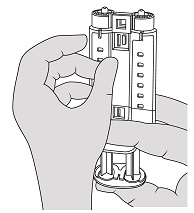
Figure 4
- Attach the Dual Applicator. See Figure 5.
NOTE: Do not depress plunger during attachment or prior to intended use because the two biologic components will pre-mix in the Airless Spray Tip, forming a fibrin clot that prevents dispensing. See Figure 6.
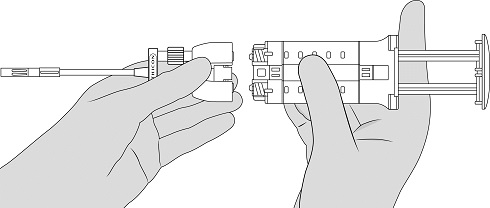
Figure 5
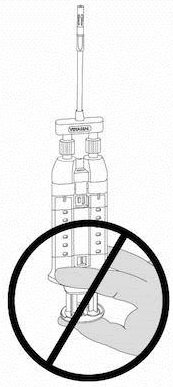
Figure 6
- Tighten luer locks and ensure the Dual Applicator is firmly attached. The device is now ready to use.
Administration
Apply VISTASEAL Fibrin Sealant (Human) using the syringe holder and plunger supplied.
Apply VISTASEAL Fibrin Sealant (Human) using the Dual Applicator provided with the product. Applicator tips cleared by the FDA for specific use with the VISTASEAL Fibrin Sealant (Human) may also be used. When using the provided Dual Applicator, follow the connection instructions in the above section for Preparation. When using other applicator tips, follow the instructions for use that are provided with the applicator tips.
Before administration of VISTASEAL Fibrin Sealant (Human):
- To prevent tissue adhesion at undesired sites, protect (cover) parts of the body outside the intended application area. [see Dosage and Administration (2.4) ]
- Use standard techniques (e.g., intermittent application of compresses, swabs, use of suction devices) to dry the surface area of the target bleeding site.
Application by spraying
- Grasp and bend the Dual Applicator to the desired position. Tip will retain its shape.
- Position the Airless Spray Tip at least 2 cm away from the target tissue. Apply firm even pressure to the plunger to spray the fibrin sealant. Increase distance accordingly to achieve desired coverage of the target area.
- If expression is stopped for any reason, change the Airless Spray Tip. To change the Airless Spray Tip, remove the device from the patient and unscrew the used Airless Spray Tip. See Figure 7. Place the used Airless Spray Tip away from the spare Airless Spray Tips. Wipe the end of the applicator using dry or moist sterile surgical gauze. Then, connect a new Airless Spray Tip provided in the package and ensure it is firmly connected before use.
NOTE: Red indicator will not be visible if Airless Spray Tip is properly connected. See Figure 8.
NOTE: Do not continue pushing the plunger in an attempt to clear the fibrin clot within the Airless Spray Tip; otherwise the applicator may become unusable.
NOTE: Do not trim the Dual Applicator to avoid exposing internal wire.
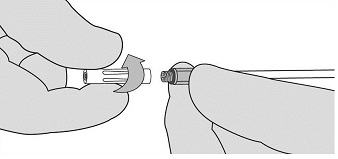
Figure 7
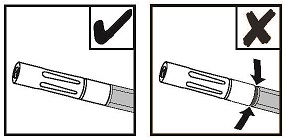
Figure 8
Application by dripping
- Remove the Airless Spray Tip portion of the spray and drip tip by unscrewing the Airless Spray Tip.
See Figure 7. - Grasp and bend the drip tip to the desired position. Tip will retain its shape.
- During dripping, keep the end of the drip tip as close to the tissue surface as possible without touching the tissue during application.
- Apply individual drops to the surface area to be treated. To prevent uncontrolled clotting, allow the drops to separate from each other and from the end of the drip tip.
NOTE: Do not reconnect a used drip tip after it has been removed from the adapter; otherwise a clot may form inside the drip tip and the applicator may become unusable.
Application precautions
- Before administration of VISTASEAL, protect (cover) parts of the body outside the desired application area to prevent tissue adhesion at undesired sites. [see Dosage and Administration (2.3)
- Apply VISTASEAL as a thin layer. Excessive clot thickness may negatively interfere with the product’s efficacy and the wound healing process. [see Dosage and Administration (2.1)]
- Only spray VISTASEAL if it is possible to accurately judge the distance from the spray tip to the tissue surface. [see Dosage and Administration (2.3)]
- No clinical data are available to support the use of this product in neurosurgery or application through a flexible endoscope for treatment of bleeding.
More about VistaSeal (fibrinogen / thrombin topical)
Professional resources
Other brands
Related treatment guides
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.









