Afstyla Dosage
Generic name: LONOCTOCOG ALFA 250[iU] in 2.5mL;
Dosage form: injection
Drug class: Miscellaneous coagulation modifiers
Medically reviewed by Drugs.com. Last updated on Jun 23, 2023.
For intravenous use after reconstitution only.
Dosing Guidelines
- Dose and duration of treatment depend on the severity of the Factor VIII deficiency, the location and extent of bleeding, and the patient's clinical condition.
- Each vial of AFSTYLA states the actual amount of Factor VIII activity in International Units (IU) as determined by chromogenic assay. One IU corresponds to the activity of Factor VIII contained in 1 milliliter (mL) of normal human plasma.
- Plasma Factor VIII levels can be monitored using either a chromogenic assay or a one-stage clotting assay – routinely used in US clinical laboratories. If the one-stage clotting assay is used, multiply the result by a conversion factor of 2 to determine the patient's Factor VIII activity level [see Warnings and Precautions (5.3)].
Calculating Required Dose
- The calculation of the required dose of Factor VIII is based on the empirical finding that 1 IU Factor VIII per kg body weight raises the plasma Factor VIII level by 2 IU/dL.
The expected in vivo peak increase in Factor VIII level expressed as IU/dL (or % of normal) is estimated using the following formula:
Estimated Increment of Factor VIII (IU/dL or % of normal) = [Total Dose (IU)/body weight (kg)] × 2 (IU/dL per IU/kg)
The dose to achieve a desired in vivo peak increase in Factor VIII level may be calculated using the following formula:
Dose (IU) = body weight (kg) × Desired Factor VIII rise (IU/dL or % of normal) × 0.5 (IU/kg per IU/dL)
- The amount of AFSTYLA to be administered and the frequency of administration should always be oriented to the clinical effectiveness in the individual case.
On-demand Treatment and Control of Bleeding Episodes
A guide for dosing AFSTYLA in the treatment and control of bleeding episodes is provided in Table 1. Consideration should be given to maintaining a Factor VIII activity at or above the target range.
| Type of Bleeding Episode | Factor VIII Activity Level Required (% or IU/dL) |
Frequency of Doses (hours) |
|---|---|---|
| Minor Uncomplicated hemarthrosis, minor muscle bleeding or oral bleeding |
20-40 | Repeat injection every 12-24 hours until the bleeding is resolved. |
| Moderate Muscle bleeding (except iliopsoas), hemarthrosis, or mild trauma |
30-60 | Repeat injection every 12-24 hours until the bleeding is resolved. |
| Major/Life-threatening Limb threatening hemorrhage, deep muscle bleeding (including iliopsoas), intracranial and retropharyngeal bleeding, fractures or head trauma |
60-100 | Repeat injection every 8-24 hours until bleed is resolved. |
Routine Prophylaxis
- Adults and adolescents (≥12 years): The recommended starting regimen is 20 to 50 IU per kg of AFSTYLA administered 2 to 3 times weekly.
- Children (<12 years): The recommended starting regimen is 30 to 50 IU per kg of AFSTYLA administered 2 to 3 times weekly. More frequent or higher doses may be required in children <12 years of age to account for the higher clearance in this age group [see Clinical Pharmacology (12.3)].
- The regimen may be adjusted based on patient response.
Perioperative Management of Bleeding
A guide for dosing AFSTYLA during surgery (perioperative management of bleeding) is provided in Table 2. Consideration should be given to maintaining a Factor VIII activity at or above the target range.
| Type of Surgery | Factor VIII Activity Level Required (% or IU/dL) |
Frequency of Doses (hours) / Duration of Therapy (days) |
|---|---|---|
| Minor (including tooth extraction) |
30-60 | Repeat injection every 24 hours for at least 1 day, until healing is achieved. |
| Major (intracranial, intra-abdominal, intrathoracic, or joint-replacement) |
80-100 | Repeat injection every 8-24 hours until adequate wound healing, then continue therapy for at least another 7 days to maintain a Factor VIII activity of 30-60% (IU/dL). |
Preparation and Reconstitution
- Reconstitute AFSTYLA using aseptic technique with diluent provided in the kit.
- Visually inspect the reconstituted solution for particulate matter prior to administration. The solution should be free from visible particles. Do not use if particulate matter is observed.
The procedures provided below are general guidelines for the preparation and reconstitution of AFSTYLA.
AFSTYLA Reconstitution Instructions
- Ensure that the AFSTYLA vial and diluent vial are at room temperature. Prepare and administer using aseptic technique.
- Place the AFSTYLA vial, diluent vial, and Mix2Vial® transfer set on a flat surface.
- Remove AFSTYLA and diluent vial flip caps. Wipe the stoppers with the sterile alcohol swab provided and allow the stoppers to dry prior to opening the Mix2Vial transfer set package.
- Open the Mix2Vial transfer set package by peeling away the lid (Fig. 1). Leave the Mix2Vial transfer set in the clear package.
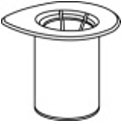
Fig. 1
- Place the diluent vial on a flat surface and hold the vial tightly. Grip the Mix2Vial transfer set together with the clear package and push the plastic spike at the blue end of the Mix2Vial transfer set firmly through the center of the stopper of the diluent vial (Fig. 2).
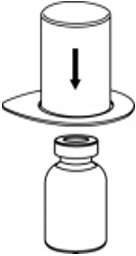
Fig. 2
- Carefully remove the clear package from the Mix2Vial transfer set. Make sure that you pull up only the clear package, not the Mix2Vial transfer set (Fig. 3).
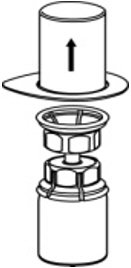
Fig. 3
- With the AFSTYLA vial placed firmly on a flat surface, invert the diluent vial with the Mix2Vial transfer set attached and push the plastic spike of the transparent adapter firmly through the center of the stopper of the AFSTYLA vial (Fig. 4). The diluent will automatically transfer into the AFSTYLA vial.
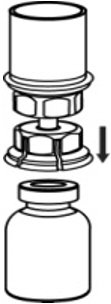
Fig. 4
- With the diluent and AFSTYLA vial still attached to the Mix2Vial transfer set, gently swirl the AFSTYLA vial to ensure that the AFSTYLA is fully dissolved (Fig. 5). Do not shake the vial.
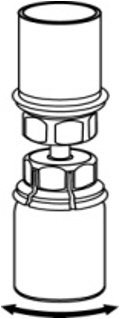
Fig. 5
- With one hand, grasp the AFSTYLA side of the Mix2Vial transfer set and with the other hand grasp the blue diluent-side of the Mix2Vial transfer set, and unscrew the set into two pieces. (Fig. 6).
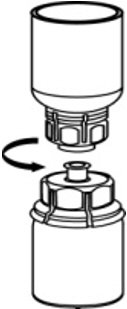
Fig. 6
- Draw air into an empty, sterile syringe. While the AFSTYLA vial is upright, screw the syringe to the Mix2Vial transfer set. Inject air into the AFSTYLA vial. While keeping the syringe plunger pressed, invert the system upside down and draw the concentrate into the syringe by pulling the plunger back slowly. (Fig. 7).
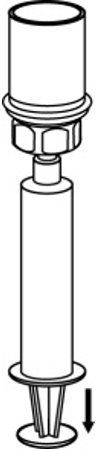
Fig. 7
- Now that the concentrate has been transferred into the syringe, firmly grasp the barrel of the syringe (keeping the plunger facing down) and unscrew the syringe from the Mix2Vial transfer set (Fig. 8).

Fig. 8
- After reconstitution, infuse immediately or within 4 hours. Reconstituted AFSTYLA may be stored at room temperature, not to exceed 25°C (77°F), for up to 4 hours. Do not freeze. Protect from direct sunlight.
- Record treatment – Remove the peel-off portion of the label from each vial used, and affix it to the patient's treatment diary/log book or scan the vial if recording the infusion electronically.
- If the dose requires more than one vial, use a separate, unused Mix2Vial® transfer set for each product vial. Repeat step 10 to pool the contents of the vial into one syringe.
Administration
- Use aseptic technique when administering AFSTYLA.
- Do not mix AFSTYLA with other medicinal products.
- Administer by intravenous injection. The rate of administration should be determined by the patient's comfort level. Do not exceed infusion rate of 10 mL per minute.
- Administer AFSTYLA at room temperature within 4 hours after reconstitution.
- AFSTYLA is for single-dose only. Following administration, discard any unused solution and all administration equipment in an appropriate manner as per local requirements.
- If a central venous access device (CVAD) is required, risk of CVAD-related complications including local infections, bacteremia and catheter site thrombosis should be considered.
More about Afstyla (antihemophilic factor)
- Check interactions
- Compare alternatives
- Pricing & coupons
- Side effects
- During pregnancy
- FDA approval history
- Drug class: miscellaneous coagulation modifiers
- Breastfeeding
- En español
Patient resources
Other brands
Advate, Altuviiio, Eloctate, Adynovate, ... +14 more
Professional resources
- Afstyla prescribing information
- Antihemophilic Factor (recombinant), PEGylated-aucl (AHFS Monograph)
Other brands
Advate, Adynovate, Altuviiio, Eloctate, ... +12 more
Related treatment guides
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.









