The originating document has been archived. We cannot confirm the completeness, accuracy, or currency of the content.
Yobine Injection
This page contains information on Yobine Injection for veterinary use.The information provided typically includes the following:
- Yobine Injection Indications
- Warnings and cautions for Yobine Injection
- Direction and dosage information for Yobine Injection
Yobine Injection
This treatment applies to the following species: Company: Lloyd
Company: Lloyd
(Yohimbine Sterile Solution)
2.0 mg/mL
Xylazine Reversing Agent and Antidote
For Use in Dogs Only
NADA # 140-866, Approved by FDA
Yobine Injection Caution
Federal law restricts this drug to use by or on the order of a licensed veterinarian.
Description
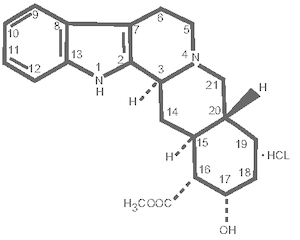
Yobine® contains yohimbine with the chemical name 17 alpha-hydroxy 20 alpha-yohimban-16 beta-carboxylic acid methyl ester. Yohimbine hydrochloride has a molecular weight of 390.89, and the molecular formula is C21H26N203•HCl. The structural formula is:
Each mL contains: Yohimbine (as the hydrochloride) 2.0 mg, methylparaben 0.9 mg, propylparaben 0.1 mg, citric acid 3.34 mg, and water for injection, pH adjusted with sodium hydroxide.
Pharmacology
Yohimbine is an indolealkylamine alkaloid that acts primarily by blocking central alpha-2 adrenoreceptors that are stimulated by xylazine. Yohimbine is an alpha-2 adrenergic receptor antagonist that easily penetrates the blood-brain barrier. It competitively blocks and antagonizes central nervous system depression or sedation and the bradycardia and respiratory depression caused by xylazine.
Xylazine, an alpha-2 adrenergic agonist with potent sedative, analgesic and muscle relaxant properties, has been used extensively as an analgesic-sedative restraining agent. It has also been used as a preanesthetic agent for many general anesthetics. The central nervous system depressant effect, as well as other pharmacologic effects, is dose dependent.
Yohimbine is useful to counteract the sedation after standard doses of xylazine. The competitive selective blocking of the alpha-2 adrenergic receptor by yohimbine displaces xylazine from these sites and thereby rapidly cancels the effect of the xylazine.
Yohimbine, when used at the prescribed dose, will effectively reverse the effects of xylazine when it is used alone in dogs. Yohimbine abbreviates the anesthesia and chemical restraint of the xylazine.
The reversal of the sedative effects of xylazine by I.V. injection of yohimbine is rapid, usually occurring within one to three minutes, regardless of the route of administration of xylazine.
Yobine Injection Indications
Yohimbine should be used in dogs when it is desirable to reverse the effects of xylazine. Yohimbine has been used successfully to reverse the sedative effects of xylazine and to reverse the cardiac effects of xylazine such as arrhythmia and bradycardia when xylazine is administered alone.
Dosage and Administration
For intravenous injection. The usual dose is 0.5 mL/20 lb body weight (0.05 mg/lb, or 0.11 mg/kg) to reverse the sedative effects of xylazine. The carefully calculated dose of yohimbine should be given intravenously slowly.Side Effects
Occasionally a dog that has been reversed will show signs of apprehensiveness but this state quickly subsides.Precautions
The safety of yohimbine in pregnant dogs or in dogs intended for breeding has not been established.Careful consideration should be given before administering to dogs known to be epileptic or seizure prone. The drug reverses the analgesic effects of xylazine as well as the sedative effects. If the animal was given xylazine for its analgesic properties, reversal may result in return of normal pain perception.
SAFETY: Yobine® was tolerated in dogs at 3 times (0.15 mg/lb) and 5 times (0.25 mg/lb) the recommended dose administered without xylazine at 3 intervals of 6 hours. Dose at the 5x magnitude occasionally produced brief seizures and muscle tremors but no lasting untoward effects were observed.
Warning
Not for human use. This drug should not be administered to food-producing animals.Storage
Protect from heat and light. Do not store over 30°C (86°F).How Supplied
20 mL multiple-dose vial.NDC 59399-115-20
References
1. Short, C.E., Ed: Principles and Practice of Veterinary Anesthesia, Williams and Wilkens, Baltimore, MD, 1987.
2. Hsu, WH. Xylazine-Induced Depression and Its Antagonism by Alpha Adrenergic Blocking Agents. The Journal of Pharmacology and Experimental Therapeutics. Vol. 218, No. 1, (188-192), 1981.
3. Hatch, RC, et al. Antagonism of Xylazine Sedation with Yohimbine, 4-Aminopyridine, and Doxapram in Dogs. American Journal of Veterinary Research. Vol. 46, No. 2, (371-375), 1985.
4. Vet-A-Mix Research
Manufactured by Akorn, Inc., Lake Forest, IL 60045
YO00N
Rev. 07/14
NAC No.: 1783005.0
1925 WEST FIELD COURT, SUITE 300, LAKE FOREST, IL, 60045
| Telephone: | 888-628-0581 | |
| Website: | www.akornanimalhealth.com |
 |
Every effort has been made to ensure the accuracy of the Yobine Injection information published above. However, it remains the responsibility of the readers to familiarize themselves with the product information contained on the US product label or package insert. |

Copyright © 2024 Animalytix LLC. Updated: 2024-02-27
