The originating document has been archived. We cannot confirm the completeness, accuracy, or currency of the content.
Tucoprim Powder
This treatment applies to the following species: Company: Zoetis
Company: Zoetis
(trimethoprim and sulfadiazine)
67 mg trimethoprim and 333 mg sulfadiazine per gram
Powder
For Use in Horses
Tucoprim Powder Caution
Federal (USA) law restricts this drug to use by or on the order of a licensed veterinarian.
Description
TUCOPRIM Powder contains 67 mg trimethoprim and 333 mg sulfadiazine per gram.
TUCOPRIM Powder is a combination of trimethoprim and sulfadiazine in the ratio of 1 part to 5 parts by weight, which provides effective antibacterial activity against a wide range of bacterial infections in animals.
The chemical structure of trimethoprim is
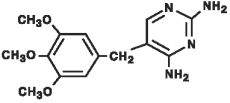
The chemical name of trimethoprim is 2,4 diamino-5-(3,4,5-trimethoxybenzyl) pyrimidine.
Actions
Microbiology
Trimethoprim blocks bacterial production of tetrahydrofolic acid from dihydrofolic acid by binding to and reversibly inhibiting the enzyme dihydrofolate reductase.
Sulfadiazine, in common with other sulfonamides, inhibits bacterial synthesis of dihydrofolic acid by competing with para-aminobenzoic acid.
Trimethoprim/sulfadiazine thus imposes a sequential double blockade on bacterial metabolism. This deprives bacteria of nucleic acids and proteins essential for survival and multiplication and produces a high level of antibacterial activity which is usually bactericidal.
Although both sulfadiazine and trimethoprim are antifolate, neither affects the folate metabolism of animals. The reasons are: animals do not synthesize folic acid and cannot, therefore, be directly affected by sulfadiazine; and although animals must reduce their dietary folic acid to tetrahydrofolic acid, trimethoprim does not affect this reduction because its affinity for dihydrofolate reductase of mammals is significantly less than for the corresponding bacterial enzyme.
Trimethoprim/sulfadiazine is active against a wide spectrum of bacterial pathogens, both gram-negative and gram-positive. The following in vitro data are available, but their clinical significance is unknown. In general, species of the following genera are sensitive to trimethoprim/sulfadiazine:
|
Very Sensitive |
Sensitive |
Moderately Sensitive |
Not Sensitive |
|
Escherichia Streptococcus Proteus Salmonella Pasteurella Shigella Haemophilus |
Staphylococcus Neisseria Klebsiella Fusiformis Corynebacterium Clostridium Bordetella |
Moraxella Nocardia Brucella |
Mycobacterium Leptospira Pseudomonas Erysipelothrix |
As a result of the sequential double blockade of the metabolism of susceptible organisms by trimethoprim and sulfadiazine, the minimum inhibitory concentration (MIC) of trimethoprim/sulfadiazine is markedly less than that of either of the components used separately. Many strains of bacteria that are not susceptible to one of the components are susceptible to the combination. A synergistic effect between trimethoprim and sulfadiazine in combination has been shown experimentally both in vitro and in vivo (in dogs).
Trimethoprim/sulfadiazine is bactericidal against susceptible strains and is often effective against sulfonamide-resistant organisms. In vitro sulfadiazine is usually only bacteriostatic.
The precise in vitro MIC of the combination varies with the ratio of the drugs present, but action of trimethoprim/sulfadiazine occurs over a wide range of ratios with an increase in the concentration of one of its components compensating for a decrease in the other. It is usual, however, to determine MICs using a constant ratio of 1 part trimethoprim in 20 parts of the combination.
The following table shows MICs using the above ratio, of bacteria which were susceptible to both trimethoprim (TMP) and sulfadiazine (SDZ). The organisms are those most commonly involved in conditions for which trimethoprim/sulfadiazine is indicated.
AVERAGE MINIMUM INHIBITORY CONCENTRATION (MIC-mcg/ml)
|
Bacteria |
TMP |
SDZ |
TMP/SDZ |
|
|
TMP |
SDZ |
|||
|
Escherichia coli |
0.31 |
26.5 |
0.07 |
1.31 |
|
Proteus species |
1.3 |
24.5 |
0.15 |
2.85 |
|
Staphylococcus aureus |
0.6 |
17.6 |
0.13 |
2.47 |
|
Pasteurella species |
0.06 |
20.1 |
0.03 |
0.56 |
|
Salmonella species |
0.15 |
61.0 |
0.05 |
0.95 |
|
β Streptococcus |
0.5 |
24.5 |
0.15 |
2.85 |
The following table demonstrates the marked effect of the trimethoprim and sulfadiazine combination against sulfadiazine resistant strains of normally susceptible organisms:
AVERAGE MINIMUM INHIBITORY CONCENTRATION OF SULFADIAZINE-RESISTANT STRAINS (MIC-mcg/ml)
|
Bacteria |
TMP Alone |
SDZ Alone |
TMP/SDZ |
|
|
TMP |
SDZ |
|||
|
Escherichia coli |
0.32 |
> 245 |
0.27 |
5.0 |
|
Proteus species |
0.66 |
> 245 |
0.32 |
6.2 |
Susceptibility Testing
In testing susceptibility to trimethoprim/sulfadiazine, it is essential that the medium used does not contain significant amounts of interfering substances which can bypass the metabolic blocking action, e.g., thymidine or thymine.
The standard SxT disc is appropriate for testing by the disc diffusion method.
Pharmacology
Following oral administration, trimethoprim/sulfadiazine is rapidly absorbed and widely distributed throughout body tissues. Concentrations of trimethoprim are usually higher in tissues than in blood. The levels of trimethoprim are high in lungs, kidneys and liver, as would be expected from its physical properties.
Serum trimethoprim concentrations in horses following oral administration indicate rapid absorption of the drug; peak concentrations occur in 1.5 hours. The mean serum elimination half-life is 2 to 2.5 hours. Sulfadiazine absorption is slower, requiring 2.5 to 6 hours to reach peak concentrations. The mean serum elimination half-life for sulfadiazine is 4 to 5.5 hours.
Usually, the concentration of an antibacterial in the blood and the in vitro MIC of the infecting organism indicate an appropriate period between doses of a drug. This does not hold entirely for trimethoprim/sulfadiazine because trimethoprim, in contrast to sulfadiazine, localizes in tissues and therefore, its concentration and ratio to sulfadiazine are higher there than in blood.
The following table shows the average concentration of trimethoprim and sulfadiazine, as measured in either serum or plasma, in 24 adult horses observed after a single dose of TUCOPRIM Powder:
AVERAGE PLASMA CONCENTRATION (mcg/ml)
|
Trimethoprim (5 mg/kg) |
Sulfadiazine (25 mg/kg) |
||||||||
|
1 hr |
3 hr |
6 hr |
10 hr |
24 hr |
1 hr |
3 hr |
6 hr |
10 hr |
24 hr |
|
0.82 |
0.69 |
0.36 |
0.12 |
< 0.25 |
9.9 |
18.8 |
17.3 |
9.0 |
1.6 |
Excretion of trimethoprim/sulfadiazine is chiefly by the kidneys, by both glomerular filtration and tubular secretion. Urine concentrations of both trimethoprim and sulfadiazine are severalfold higher than blood concentrations. Neither trimethoprim nor sulfadiazine interferes with the excretion pattern of the other.
Tucoprim Powder Indications And Usage
Trimethoprim/sulfadiazine is indicated in horses where potent systemic antibacterial action against sensitive organisms is required. Trimethoprim/sulfadiazine is indicated where control of bacterial infections is required during treatment of:
Acute Strangles
Acute Urogenital Infections
Respiratory Tract Infections
Wound Infections and Abscesses
Trimethoprim/sulfadiazine is well tolerated by foals.
Contraindications
Trimethoprim/sulfadiazine should not be used in horses showing marked liver parenchymal damage, blood dyscrasias or in those with a history of sulfonamide sensitivity.
Warnings
NOT FOR HUMAN USE. KEEP OUT OF REACH OF CHILDREN.
Do not use in horses intended for human consumption.
Precaution
Water should be readily available to horses receiving sulfonamide therapy.
Adverse Reactions
During clinical trials, one case of anorexia and one case of loose feces following treatment with the drug were reported.
Individual animal hypersensitivity may result in local or generalized reactions, sometimes fatal. Anaphylactoid reactions, although rare, may also occur. Antidote: Epinephrine.
Post Approval Experience
Horses have developed diarrhea during trimethoprim/sulfadiazine treatment, which could be fatal. If fecal consistency changes during trimethoprim/sulfadiazine therapy, discontinue treatment immediately and contact your veterinarian.
Animal Safety
Toxicity is low. The acute toxicity (LD50) of trimethoprim/sulfadiazine is more than 5 g/kg orally in rats and mice. No significant changes were recorded in rats given doses of 600 mg/kg per day for 90 days.
Horses treated intravenously with trimethoprim/sulfadiazine 48% Injection have tolerated up to five times the recommended daily dose for 7 days or on the recommended daily dose for 21 consecutive days without clinical effects or histopathological changes.
Lengthening of clotting time was seen in some of the horses on high or prolonged dosing in one of two trials. The effect, which may have been related to a resolving infection, was not seen in a second similar trial.
Slight to moderate reductions in hematopoietic activity following high, prolonged dosage in several species have been recorded. This is usually reversible by folinic acid (leucovorin) administration or by stopping the drug. During long-term treatment of horses, periodic platelet counts and white and red blood cell counts are advisable.
In rare instances, horses have developed diarrhea during trimethoprim/sulfadiazine treatment. If fecal consistency changes during trimethoprim/sulfadiazine therapy, discontinue treatment immediately and institute appropriate symptomatic measures.
Teratology
The effect of trimethoprim/sulfadiazine on pregnancy has not been determined. Studies to date show there is no detrimental effect on stallion spermatogenesis with or following the recommended dose of trimethoprim/sulfadiazine.
Tucoprim Powder Dosage And Administration
The recommended dose is 3.75 grams TUCOPRIM Powder per 50 kg (110 lbs) body weight per day. Each level, loose-filled scoop contains approximately 15 grams which is sufficient to treat 200 kg (440 lbs) of body weight. Since product contents may settle, gentle agitation during scooping is recommended. Administer orally once a day in a small amount of palatable feed.
The usual course of treatment is a single, daily dose for 5 to 7 days. Continue acute infection therapy for 2 or 3 days after clinical signs have subsided. If no improvement of acute infections is seen in 3 to 5 days, re-evaluate the diagnosis.
Trimethoprim/sulfadiazine may be used alone or in conjunction with intravenous dosing. Following treatment with trimethoprim/sulfadiazine 48% Injection, therapy can be maintained using oral powder.
A complete blood count should be done periodically in patients receiving trimethoprim/sulfadiazine for prolonged periods. If significant reduction in the count of any formed blood element is noted, treatment with trimethoprim/sulfadiazine should be discontinued.
Storage Conditions
Store at or below 30°C.
How Supplied
TUCOPRIM Powder is available in the following package sizes:
400 gram bottle
2000 gram pails
ANADA #200-244, Approved by FDA
Made in China
Distributed by: Zoetis Inc., Kalamazoo, MI 49007
Revised: January 2013
|
400 grams |
1519000 A73050-4 |
|
2000 grams |
1494000 A73051-4 |
CPN: 3690480.1
333 PORTAGE STREET, KALAMAZOO, MI, 49007
| Telephone: | 269-359-4414 | |
| Customer Service: | 888-963-8471 | |
| Website: | www.zoetis.com |
 |
THIS SERVICE AND DATA ARE PROVIDED "AS IS". DVMetrics assumes no liability, and each user assumes full risk, responsibility, and liability, related to its use of the DVMetrics service and data. See the Terms of Use for further details. |

Copyright © 2025 Animalytix LLC. Updated: 2024-11-27
