Semintra Oral Solution 4 mg/mL (Canada)
This treatment applies to the following species: Company: Boehringer Ingelheim Animal Health
Company: Boehringer Ingelheim Animal Health
4 mg/mL
Oral Solution
Telmisartan Oral Solution
Veterinary Use Only
For Cats
DIN 02418126
Description
Each mL contains 4 mg telmisartan, Ph. Eur. in a clear, colourless to yellowish viscous solution and 0.1 mg benzalkonium chloride, Ph. Eur., as a preservative.
Semintra® Oral Solution 4 mg/mL is a non-peptide angiotensin II receptor (type AT1) antagonist. This class of drug is also referred to as Angiotensin Receptor Blockers (ARBs).
Semintra Oral Solution 4 mg/mL Indications
Reduction of proteinuria associated with chronic kidney disease (CKD) in cats.
Dosage and Administration
Semintra® should be administered once daily at a dosage of 1 mg telmisartan/kg body weight (equivalent to 0.25 mL/kg body weight) either directly into the mouth or with a small amount of food. Semintra® is well accepted by most cats.
The solution should be given using the measuring syringe provided in the package. The syringe fits onto the bottle and has a kg-body weight scale.
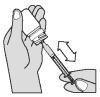
Push down and turn cap, to open the bottle. Attach the dosing syringe to the plug-in adapter of the bottle by gently pushing.
Turn the bottle/syringe upside down. Pull the plunger out until the end of the plunger corresponds to your cat’s body weight in kilograms. Separate the dosing syringe from the bottle.
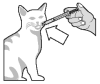
Push the plunger to empty the contents of the syringe directly into the mouth of the cat ...
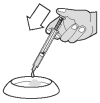
... or onto a small amount of food.
After administration, close bottle tightly with cap. To avoid contamination, use the provided syringe only to administer Semintra®.
Upon opening, use within 6 months.
Contraindications
Semintra® should not be used in case of hypersensitivity to the active substance or to any of the excipients.
Interaction with other medicinal products: In a field efficacy trial, concomitant therapy with amlodipine at the recommended dose was well tolerated and no clinical signs of hypotension were seen.
Semintra Oral Solution 4 mg/mL Caution
The safety of Semintra® has not been evaluated in breeding, pregnant or lactating cats, cats less than 9 months of age.
The safety of Semintra® has not been evaluated in cats with acute renal failure, acute exacerbation of chronic renal failure, hypovolemia, dehydration, diabetes or concurrent cardiac or hepatic disease. Due to the mode of action of the veterinary medicinal product, transient hypotension may occur.
Symptomatic treatment, e.g. fluid therapy, should be provided in case of any clinical signs of hypotension.
As known from substances acting on the Renin-Angiotensin-Aldosterone System (RAAS), such as Angiotensin Receptor Blockers (ARBs) and ACE inhibitors (ACEi), a decrease in red blood cell count may occur. Red blood cell count should be monitored during therapy.
Warning: Keep out of reach of children.
In case of accidental ingestion, seek medical advice immediately and show the package insert or the label to the physician. Avoid eye contact. In case of such contact, rinse eyes with water. Wash hands after use.
People with hypersensitivity to telmisartan or other angiotensin II receptor antagonists should avoid contact with Semintra®.
Pregnant women should take special care to avoid accidental oral exposure because substances acting on the RAAS, such as Angiotensin Receptor Blockers (ARBs) and ACE inhibitors (ACEi) have been found to affect the unborn child during pregnancy in humans.
Adverse Reactions
The following transient gastrointestinal signs have rarely been observed in a field efficacy trial (in order of decreasing frequency): mild and intermittent regurgitation, vomiting, diarrhea or soft feces.
Elevated liver enzymes have been very rarely observed and values normalized following cessation of therapy.
Effects attributable to the pharmacological activity of the product observed at the recommended treatment dose included reductions in blood pressure and slight decreases in red blood cell counts. If you notice any serious effects or other effects not mentioned in the package insert, please inform your veterinarian.
Pharmacology
a) Mechanism of Action:
Telmisartan is an orally active and specific angiotensin II receptor (type AT1) antagonist.
Telmisartan displaces angiotensin II with very high affinity from its binding site at the AT1 receptor subtype. Telmisartan selectively binds to the AT1 receptor and does not show affinity for other receptors, including AT2 or other less characterized AT receptors. Stimulation of the AT1 receptor is responsible for most of the pathologic effects of angiotensin II in the kidney and other organs such as vasoconstriction, retention of sodium and water, increased aldosterone synthesis and organ remodelling. By selectively binding to only the AT1 receptor the beneficial effects associated with stimulation of the AT2 receptor such as vasodilatation, natriuresis and inhibition of inappropriate cell growth are not suppressed. The receptor binding is long lasting due to the slow dissociation of telmisartan from the AT1 receptor binding site. Telmisartan does not exhibit any partial agonist activity at the type AT1 receptor.
b) Pharmacodynamics:
Telmisartan causes a dose dependent decrease in mean arterial blood pressure in mammalian species including the cat. In a clinical trial in cats with chronic kidney disease, a reduction in proteinuria was seen in the first 7 days after the start of treatment. Hypokalemia is known to be commonly associated with CKD, however telmisartan did not affect serum potassium levels in the clinical field trial in cats.
c) Pharmacokinetics:
Absorption:
Following oral administration of 1.0 mg/kg body weight telmisartan to cats, plasma-concentration-time curves of the parent compound are characterized by rapid absorption, with maximum plasma concentrations (Cmax) achieved after 0.5 hours (tmax). For both, Cmax-values and AUC-values a dose proportional increase over the dose range from 0.5 mg to 3 mg/kg was observed. As determined by AUC, food consumption does not affect the overall extent of absorption of telmisartan.
Telmisartan is highly lipophilic and has rapid membrane permeability kinetics, which facilitates easy distribution into tissue. No significant gender effect was seen. No clinically relevant accumulation was observed following multiple dose administration once daily for 21 days.
The absolute bioavailability after oral administration was found to be 33%.
Distribution:
Telmisartan is highly bound to plasma proteins (> 99.5%), mainly to albumin and α-1-acid glycoprotein.
Metabolism:
Telmisartan is metabolized by conjugation to form a pharmacologically inactive metabolite (1-O-acylglucuronide). From in vitro and ex vivo studies with feline liver microsomes it can be concluded that telmisartan is effectively glucuronidated in the cat.
Elimination:
The terminal elimination half-life (t 1/2) ranged from 7.3 hours to 8.6 hours, with mean value 7.7 hours. After oral administration, telmisartan is almost exclusively eliminated in the feces via biliary excretion.
Safety Study:
A 6 month laboratory margin of safety study was conducted to evaluate the safety of telmisartan oral solution when administered once daily for 6 months to healthy adult cats at 0x, 1x, 3x and 5x the maximum daily target dose of 1 mg/kg.
There were no drug-related negative effects on survival, cage-side observations, detailed physical examinations, veterinary examinations, body weights, ophthalmology examinations, coagulation parameters, urinalysis, or macroscopic gross necropsy examinations.
Expected pharmacological effects of lower blood pressure were noted throughout the study beginning during study week 4, 2 and 0 in the 1x, 3x and 5x groups, respectively, when compared with the control group.
Administration of telmisartan to cats for six months in the 1x, 3x and 5x groups, respectively, was not associated with any drug-related differences in group mean bone marrow cytology values. Alterations in erythroid cell relative populations were noted in some cats in the 5x telmisartan dose group and were considered potentially drug-related due to concurrent observations of reductions in peripheral erythroid mass.
Drug-related effects on hematology parameters included lower red blood cells, hemoglobin, hematocrit and reticulocytes in the 3x and 5x groups compared to control cats.
Drug-related effects on serum chemistry parameters consisted of higher blood urea nitrogen (BUN) in the 3x and 5x groups compared to control cats.
Drug-related alterations in the kidney were limited to minimal to mild hypertrophy of the juxtaglomerular apparatus (JGA) in the 1x, 3x and 5x groups, respectively.
Drug-related changes in the stomach included minimal ulceration on histological examination in one 5x cat.
Vomiting was observed more frequently in cats in the 3x and 5x treatment groups.
Efficacy Study:
A positive controlled, randomized, single blinded, multi-site field efficacy study [in six European countries] was conducted with the objective to show non-inferiority of telmisartan compared to benazepril for the reduction of proteinuria (measured as UPC ratio) in cats with CKD (Chronic Kidney Disease).
The study included 224 cats presenting with CKD, IRIS stage IIa to IV and urine specific gravity (USG) <1.035 but no evidence of other comorbid conditions. Cats with IRIS stage IV were eventually excluded from participation in the study. Cats could be included if they had moderate hypertension (<180 mmHg) providing that they were stabilized on conjunct amlodipine treatment first. Approximately 30% of cats in both groups were fed a commercial kidney diet when being allocated to the study.
Primary and secondary efficacy analysis was based on the per-protocol-population data set, including 176 valid cases (n=85 telmisartan, n=91 benazepril). Both treatments showed a decrease in proteinuria during a six month observation period. The anti-proteinuric effect of telmisartan was non-inferior to benazepril as assessed based on the 2-sided 95% confidence interval approach.
The overall mean UPC values at 180 days decreased in both groups when compared to baseline with -0.02 for benazepril and -0.05 for telmisartan. Reduction in proteinuria was rapid in onset (day 7) in both treatment groups.
The incidence of reported adverse events was similar between treatment groups. The most frequently reported adverse events were common to the clinical symptomatology of CKD and considered unlikely to be treatment related by the investigators.
Telmisartan was generally well tolerated during the course of the study and no adverse reactions were reported in cats receiving combined treatment with amlodipine. Elevated liver enzymes were very rarely observed in the telmisartan treatment group and values normalised within a few days following cessation of therapy.
Storage
Store from 5 - 30°C. Upon opening, use within 6 months.Presentation: 30 mL bottle
Boehringer Ingelheim Animal Health Canada Inc., 5180 South Service Road, Burlington ON L7L 5H4
Revised: 01-2020
Semintra® is a registered trademark of Boehringer Ingelheim Vetmedica GmbH, used under licence.
159587/002
CPN: 1182169.0
5180 SOUTH SERVICE ROAD, BURLINGTON, ON, L7L 5H4
| Customer Care No.: | 1-800-567-1885 | |
| Technical Services No.: | 1-877-565-5501 | |
| Website: | www.boehringer-ingelheim.ca |
 |
THIS SERVICE AND DATA ARE PROVIDED "AS IS". Animalytix assumes no liability, and each user assumes full risk, responsibility, and liability, related to its use of the Animalytix service and data. See the Terms of Use for further details. |

Copyright © 2025 Animalytix LLC. Updated: 2025-03-02
