Wilzin Prescribing Information
Package insert / product label
Generic name: zinc acetate dihydrate
Dosage form: capsule
Drug class: Minerals and electrolytes
Medically reviewed by Drugs.com. Last updated on Jul 31, 2023.
HEALTHCARE PROVIDER LETTER

IMPORTANT PRESCRIBING INFORMATION
December 11, 2020
Teva Administrative Offices:
Teva Pharmaceuticals USA, Inc.
Morris Corporate Center
400 Interpace Parkway
Parsippany, NJ 07054
Phone: 1-888-838-2872
| Subject: Supply of GALZIN® (Zinc Acetate) 25 mg Capsules to Address Drug Shortage |
Dear Healthcare Professional:
In order to alleviate a critical shortage of GALZIN® (zinc acetate) 25 mg and 50 mg capsules in the United States (U.S.) market for the treatment of Wilson’s disease, Teva Pharmaceuticals USA Inc. (Teva) is coordinating with the U.S. Food and Drug Administration (FDA) to make available in the U.S. the non-FDA approved pharmaceutically equivalent alternative drug, WILZIN® (zinc acetate dihydrate) 25 mg capsules, lot# 30062451, expiration date 10/2022, and lot# 30062461, expiration date 10/2022, for the duration of the shortage and at no cost to patients. The zinc acetate dihydrate capsules marketed in Europe under the brand name WILZIN® by Recordati Rare Diseases are manufactured at the same facility in the U.S. that manufactures GALZIN® which is an FDA-inspected facility that complies with current Good Manufacturing Practice requirements.
At this time, no other entity except Teva is authorized by the FDA to import or distribute Recordati’s WILZIN® (zinc acetate dihydrate) capsules in the U.S. However, this does not represent a formal FDA approval of Recordati’s WILZIN® in the United States. Teva is also working to restock GALZIN® 25 mg and 50 mg capsules in the U.S. market with an anticipated restock date in February 2021.
Effective immediately, Teva will distribute the following presentation of Recordati’s WILZIN® (zinc acetate dihydrate) capsules to address the critical shortage:
| Product Name | Quantity | Capsule Description | Marketing Authorization Number(s) | NDC |
| Wilzin® (zinc acetate dihydrate) capsule, 25 mg | Bottle of 250 capsules | Aqua blue opaque cap and body, imprinted “93-376” | EU/1/04/286/001 (Europe) | 57844-0376-25 |
The bottle label will display the text used and approved for marketing the products in Europe with both English and multi-lingual translations. It is important to note that there are differences in the markings on the 25 mg capsules, as well as the format and content of the labeling between the U.S. approved product and WILZIN® capsules. Please see the product comparison tables (Appendix 1 & 2) at the end of this letter.
Zinc acetate dihydrate 25 mg capsules are available only by prescription in the U.S. Ensure that your staff and others in your office and/or pharmacy who may be involved in the prescribing and/or dispensing of zinc acetate dihydrate 25 mg capsules receive a copy of this letter, review the information and instruct patients on the differences between GALZIN® and WILZIN®. Given the differences in labeled dosing instructions between the two drugs, it is also important to note the following:
- GALZIN® is indicated in the U.S. for the “maintenance treatment of patients with Wilson’s disease who have been initially treated with a chelating agent.” However, WILZIN® is indicated in Europe for the “treatment of Wilson’s disease.”
- The recommended adult dose of GALZIN® in the U.S. is 50 mg three times daily; the maximum dose of WILZIN® in Europe is 50 mg five times daily.
- In the U.S., GALZIN® is recommended in children 10 years and older. In Europe, WILZIN® is recommended in children one year and older.
- GALZIN® capsules should be swallowed whole, not opened or chewed. WILZIN® capsules can be opened and their contents suspended in a little water for children who are unable to swallow capsules.
Please refer to the package insert for FDA-approved GALZIN® for full prescribing information available at www.GALZIN.com and follow the instructions presented in the FDA-approved package insert for indication and usage, dosage and administration. Patients requiring a 50 mg dose of zinc acetate capsules should be prescribed and dispensed an appropriate quantity based on total daily dosage and instructed to take two 25 mg capsules at one time.
There is no barcode on this product for use with U.S. barcode scanning systems. Alternative procedures should be followed to assure that the correct drug product is being used and administered to individual patients.
In addition, the packaging of the WILZIN® capsules does not include serialization information and does not meet the product identifier requirements of section 582(b)(2) of the Federal Food, Drug and Cosmetic Act.
To alleviate issues associated with reimbursement for this product without the appropriate U.S. coding available, Teva will provide WILZIN® at no cost until either reimbursement becomes available or the GALZIN® brand is available for prescription again in the US.
If you have any questions about the information contained in this letter, any quality related problems, or questions on the use of WILZIN ® (zinc acetate dihydrate) 25 mg capsules, please contact Teva Pharmaceuticals USA, Inc. at 1-888-838-2872.
To place an order, please see enclosed instructions on how to obtain the WILZIN® product (Appendix 3) or contact Teva directly at 1-888-838-2872.
Healthcare providers should report adverse events associated with the use of WILZIN® capsules to Teva at 1-888-838-2872, Option 3 and then Option 4.
Adverse events, medication errors or quality problems experienced with the use of this product may also be reported to the FDA’s MedWatch Adverse Event Reporting Program either online, by regular mail, or by fax:
- Complete and submit the report Online: www.fda.gov/medwatch/report.htm
- Regular mail or Fax: Download form www.fda.gov/MedWatch/getforms.htm or call 1-800-332-1088 to request a reporting form, then complete and return to the address on the pre-addressed form or submit by fax to 1-800-FDA-0178 (1-800-332-0178).
We remain at your disposal to answer any questions you may have about our product and to provide more information if needed.
Sincerely,
Denisa Hurtukova, MD
Vice President, Head of North America Medical Affairs
Teva Pharmaceuticals
Enclosures:
GALZIN® (zinc acetate) capsules prescribing information
Appendix 1 – Product Label and Product Characteristics Side-by-Side Comparison Table
Appendix 2 – Prescribing Information Side-by-Side Comparison Table
Appendix 3 – Instructions on How to Obtain WILZIN® as Alternate Supply for GALZIN® and Supply Request Form
Appendices are also available at www.GALZIN.com
1 The approved lot numbers (3006245 and 3006246) for the bulk product and the finished product will have an alpha character designating the finished packaged product based on the packaging run (i.e., 3006245 may be packaged as 3006245A and 3006245B; and 3006246 as 3006246A and 3006246B).
APPENDIX 1
Product Label and Product Characteristics Side-by-Side Comparison Table
| US FDA Approved Product | Import Product | |
| Product Name | GALZIN® (zinc acetate) capsules | WILZIN® (zinc acetate dihydrate) capsules |
| Bottle Container Label (main panel*) – 25 mg |  |  |
| 25 mg Capsule Images |  |  |
| Product Name | GALZIN® 25 mg (zinc acetate) capsules | WILZIN® 25 mg hard (zinc acetate dihydrate) capsules |
| Route of Administration | Oral | Oral |
| Ingredients | GALZIN® capsules contain the equivalent of 25 mg of zinc, in addition to corn starch and magnesium stearate in gelatin capsules. The 25 mg capsule shells contain titanium dioxide and FD&C Blue #1 | WILZIN® contains 25 mg of zinc (corresponding to 83.92 mg of zinc acetate dihydrate, in addition to maize starch and magnesium stearate in gelatin capsules. The 25 mg capsule shells contain titanium dioxide (E171) and brilliant blue FCF (E133). The printing ink contains black iron oxide (E172) and shellac. |
| Storage Conditions | Store at 25°C (77°F); excursions permitted to 15-30°C (59-86°F). See USP Controlled Room Temperature. Dispense in a tight, light-resistant container as defined in the USP, with a child-resistant closure. | Do not store above 25°C. |
|
* Note: The WILZIN bottle label contains a fold-out panel with multilingual text as shown below. 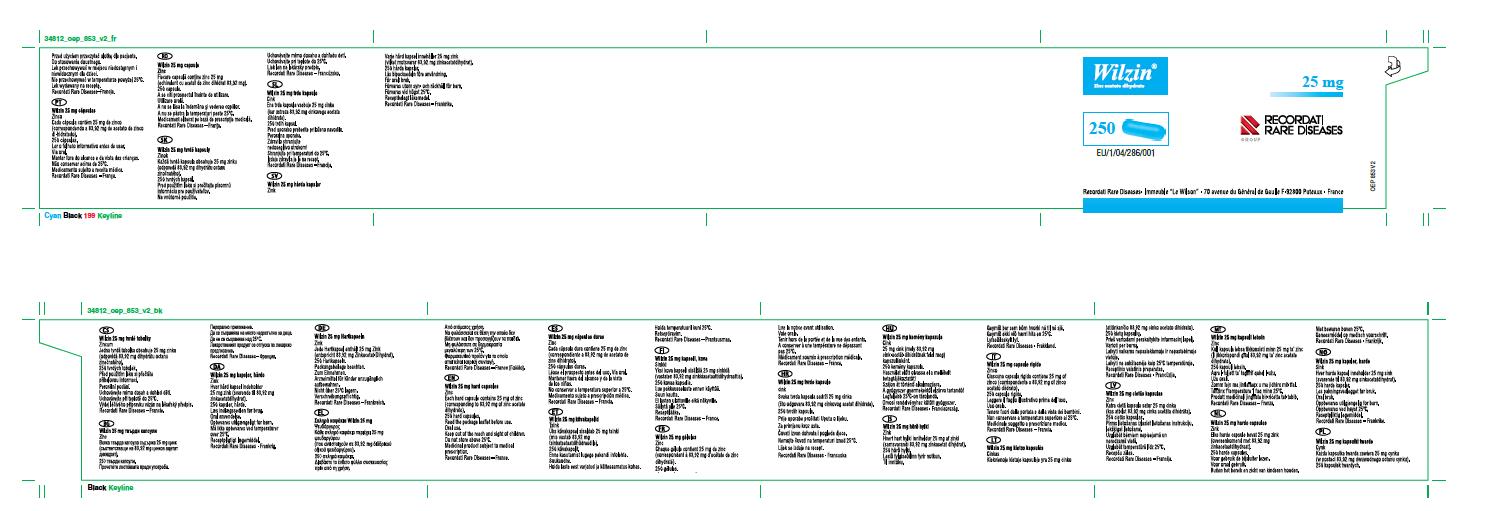 |
||
APPENDIX 2
Prescribing Information Side-by-side Comparison Table
Introduction
A side-by-side comparison of the GALZIN® U.S. Prescribing Information (USPI) and WILZIN® Summary of Product Characteristics (SmPC) is provided below.
It is important to note the following differences between the GALZIN® and WILZIN® labeling and to prescribe use of the product in the United States in accordance with the GALZIN USPI:
- GALZIN® is indicated for the “maintenance treatment of patients with Wilson’s disease who have been initially treated with a chelating agent.” However, WILZIN® is indicated for the “treatment of Wilson’s disease.”
- The recommended adult dose of GALZIN® is 50 mg three times daily; the maximum dose of WILZIN® is 50 mg five times daily.
- GALZIN® is recommended in children 10 years and older. WILZIN® is recommended in children one year of age and older.
- GALZIN® capsules should be swallowed whole, not opened or chewed. WILZIN® capsules can be opened and their contents suspended in a little water for children who are unable to swallow capsules.
- When switching a patient from chelating treatment to maintenance treatment with GALZIN®, patients may be continued on initial therapy as clinically indicated. When switching a patient from chelating treatment to maintenance treatment with WILZIN®, the chelating treatment should be maintained and co-administered for 2 to 3 weeks.
- GALZIN® capsules are aqua blue opaque cap and body imprinted with “93-215”. WILZIN® capsules are aqua blue opaque cap and body imprinted with “93-376”.
- GALZIN® capsules are stored at 25°C (77°F); excursions permitted between 15-30°C (59-86°F). WILZIN® capsules should not be stored above 25°C.
- GALZIN® is not distributed with a patient information leaflet. However, WILZIN® is provided with a “PACKAGE LEAFLET: INFORMATION FOR THE USER”.
Side-by-Side Comparison Table
| US FDA Approved Product | Import Product | |
| GALZIN® (zinc acetate) capsules | WILZIN® (zinc acetate dihydrate) capsules | |
| Product Name | GALZIN® (Zinc Acetate) Capsules | Wilzin 25 mg hard capsules 1. Name of the medicinal product Wilzin 25 mg hard capsules |
| Indication | INDICATIONS AND USAGE Zinc acetate therapy is indicated for maintenance treatment of patients with Wilson’s disease who have been initially treated with a chelating agent (See PRECAUTIONS: Monitoring Patients). | 4.1 Therapeutic indications Treatment of Wilson's disease. |
| Dosage and Administration | DOSAGE AND ADMINISTRATION
The recommended adult dose is 50 mg as zinc three times daily (See CLINICAL TRIALS). Since 25 mg t.i.d. is also an effective dose in children 10 years of age or older or in women who are pregnant, it may be advisable to use a dose of zinc to 25 mg three times a day, as long as the patient is compliant with therapy. The dose can be raised to 50 mg t.i.d. if monitoring indicates a lessening of control (see PRECAUTIONS: Monitoring Patients). Patients should take zinc acetate on an empty stomach, at least one hour before or two to three hours after meals. For additional information, see PRECAUTIONS. | 4.2 Posology and method of administration
Wilzin treatment should be initiated under the supervision of a physician experienced in the treatment of Wilson's disease (see section 4.4). Wilzin is a life-long therapy. There is no difference in dose between symptomatic and presymptomatic patients. Wilzin is available in hard capsules of 25 mg or 50 mg.
Data are very limited in children under 6 years but since the disease is fully penetrant, prophylactic treatment should be considered as early as possible. The recommended dose is as follows: - from 1 to 6 years: 25 mg twice daily
A dose of 25 mg 3 times daily is usually effective but the dose should be adjusted to copper levels (see section 4.4 and section 4.6). In all cases, dose should be adjusted according to therapeutic monitoring (see section 4.4.). Wilzin must be taken on an empty stomach, at least 1 hour before or 2-3 hours after meals. In case of gastric intolerance, often occurring with the morning dose, this dose may be delayed to mid-morning, between breakfast and lunch. It is also possible to take Wilzin with a little protein, such as meat (see section 4.5). In children who are unable to swallow capsules, these should be opened and their content suspended in a little water (possibly sugar or syrup flavoured water). When switching a patient on chelating treatment to Wilzin for maintenance therapy, the chelating treatment should be maintained and co-administered for 2 to 3 weeks since this is the time it takes for the zinc treatment to induce maximum metallothionein induction and full blockade of copper absorption. The administration of the chelating treatment and Wilzin should be separated by at least 1 hour. |
| Description | DESCRIPTION
Zinc acetate as the dihydrate is a salt of zinc used to inhibit the absorption of copper in patients with Wilson's disease. Its structural formula is: 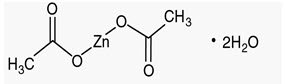 C4H6O4Zn•2H2O M.W. 219.51. Zinc acetate occurs as white crystals or granules, freely soluble in water and in boiling alcohol, and slightly soluble in alcohol. GALZIN® (Zinc Acetate) Capsules contain the equivalent of 25 or 50 mg of zinc, in addition to corn starch and magnesium stearate in gelatin capsules. The 25 mg capsule shells contain titanium dioxide and the 50 mg capsule shells contain titanium dioxide, methylparaben and propylparaben. The 25 mg capsule shells contain FD&C Blue #1; the 50 mg capsule shells contain FD&C Red #40, D&C Red #28, and D&C Yellow #10.
HOW SUPPLIED
GALZIN®, Zinc Acetate Capsules (25 mg zinc content) are #1 capsules with aqua blue opaque cap and body, imprinted "93-215." Packaged in bottles of 250 (NDC 57844-215-52). GALZIN® Zinc Acetate Capsules (50 mg zinc content) are #1 capsules with orange opaque cap and body, imprinted "93-208." Packaged in bottles of 250 (NDC 57844-208-52). Store at 25°C (77°F); excursions permitted to 15-30°C (59-86°F). See USP Controlled Room Temperature. Dispense in a tight, light-resistant container as defined in the USP, with a child-resistant closure. | 2. Qualitative and quantitative composition
Each hard capsule contains 25 mg of zinc (corresponding to 83.92 mg of zinc acetate dihydrate). For a full list of excipients, see section 6.1. 3. Pharmaceutical form Hard capsule. Capsule with aqua blue opaque cap and body, imprinted "93-376”. 6.1 List of excipients Capsule content maize starch magnesium stearate Capsule shell gelatin titanium dioxide (E171) brilliant blue FCF (E133) Printing ink black iron oxide (E172) shellac 6.2 Incompatibilities Not applicable. 6.3 Shelf life 3 years. 6.4 Special precautions for storage Do not store above 25°C. 6.5 Nature and contents of container White HDPE bottle with a polypropylene and HDPE closure and contains a filler (cotton coil). Each bottle contains 250 capsules. |
| Clinical Pharmacology | CLINICAL PHARMACOLOGY
Introduction The disease has been treated by restricting copper in the diet, and the use of chelating agents to bind free copper to reduce its toxicity and facilitate its excretion. The purpose of initial treatment of symptomatic patients with a chelating agent is to detoxify copper. Once the patient's symptoms have stabilized clinically, maintenance treatment begins. Clinical measures are used to determine whether the patient remains stable (See PRECAUTIONS: Monitoring Patients). The active moiety in zinc acetate is zinc cation. Regardless of the ligand, zinc blocks the intestinal absorption of copper from the diet and the reabsorption of endogenously secreted copper such as that from the saliva, gastric juice and bile. Zinc induces the production of metallothionein in the enterocyte, a protein that binds copper thereby preventing its serosal transfer into the blood. The bound copper is then lost in the stool following desquamation of the intestinal cells.
Pharmacokinetics In pharmacodynamic studies, the methods used included net copper balance and radiolabeled copper uptake in Wilson’s disease patients. These studies showed that a regimen of 50 mg t.i.d. of zinc acetate was effective in inducing a negative mean copper balance (-0.44 mg/day) and an adequate mean 64Cu uptake (0.82% of the administered dose). A regimen of 25 mg t.i.d. of zinc acetate was also pharmacodynamically active but fewer patients have been treated with this regimen than 50 mg t.i.d. | 5. Pharmacological properties
5.1 Pharmacodynamic properties Pharmacotherapeutic group: various alimentary tract and metabolism products, ATC code: A16AX05. Wilson's disease (hepatolenticular degeneration) is an autosomal recessive metabolic defect in hepatic excretion of copper in the bile. Copper accumulation in the liver leads to hepatocellular injury and eventual cirrhosis. When the liver capacity of storing copper is exceeded copper is released into the blood and is taken up in extra hepatic sites, such as the brain, resulting in motor disorders and psychiatric manifestations. Patients may present clinically with predominantly hepatic, neurologic, or psychiatric symptoms. The active moiety in zinc acetate dihydrate is zinc cation, which blocks the intestinal absorption of copper from the diet and the reabsorption of endogenously secreted copper. Zinc induces the production of metallothionein in the enterocyte, a protein that binds copper thereby preventing its transfer into the blood. The bound copper is then eliminated in the stool following desquamation of the intestinal cells. Pharmacodynamic investigations of copper metabolism in patients with Wilson's disease included determinations of net copper balance and radiolabelled copper uptake. A daily regimen of 150 mg of Wilzin in three administrations was shown to be effective in significantly reducing copper absorption and inducing a negative copper balance.
5.2 Pharmacokinetic properties Zinc is absorbed in the small intestine and its absorption kinetics suggest a tendency to saturation at increasing doses. Fractional zinc absorption is negatively correlated with zinc intake. It ranges from 30 to 60% with usual dietary intake (7-15 mg/d) and decreases to 7% with pharmacological doses of 100 mg/d. In the blood, about 80% of absorbed zinc is distributed to erythrocytes, with most of the remainder being bound to albumin and other plasma proteins. The liver is the main storage for zinc and hepatic zinc levels are increased during maintenance therapy with zinc. The plasma elimination half-life of zinc in healthy subjects is around 1 hour after a dose of 45 mg. The elimination of zinc results primarily from faecal excretion with relatively little from urine and sweat. The faecal excretion is in the greatest part due to the passage of unabsorbed zinc but it is also due to endogenous intestinal secretion. |
| Contra-indications | CONTRAINDICATIONS
Zinc Acetate Capsules are contraindicated in patients with known hypersensitivity to any of the components of the formulation. | 4.3 Contraindications
Hypersensitivity to the active substance or to any of the excipients. |
| Precautions | PRECAUTIONS General Zinc acetate is not recommended for the initial therapy of symptomatic patients because of the delay required for zinc-induced increase in enterocytic metallothionein and blockade of copper uptake. Symptomatic patients should be treated initially, using chelating agents. During initial therapy, neurological deterioration may occur as stores of copper are mobilized. Once initial therapy has been completed, and the patient is clinically stable, maintenance treatment with zinc acetate can be considered, but patients may be continued on initial therapy as clinically indicated.
Information for Patients Monitoring Patients Patients should be monitored primarily by assessment of existing signs and symptoms of Wilson’s disease and 24-hour urine copper. Neuropsychiatric evaluations including speech as well as liver function tests including bilirubin and aminotransferases, should be done as appropriate. The urinary excretion of copper is an accurate reflection of the body status of copper when patients are not on chelation therapy. The clinician should be aware that urinary copper levels are usually increased with chelation therapy such as penicillamine or trientine. Adequate zinc therapy will eventually decrease urinary copper excretion to 125 μg per 24 hours or less. A significant trend upward indicates impending loss of copper control. The non-ceruloplasmin plasma copper (also known as free copper) is obtained by subtracting the ceruloplasmin-bound copper from the total plasma copper. Each mg of ceruloplasmin contains 3 μg of copper. In the United States study, non-ceruloplasmin plasma copper concentration was kept below 20 μg/dL. Urine and plasma for copper determinations should be collected in copper-free containers and assayed with equipment capable of accurately measuring copper at levels as low as 0.01 μg/mL. An additional monitoring tool, if available, is the amount of radioactivity measured in the plasma 1 or 2 hours after orally administered 64copper. In adequately controlled patients, the amount is less than 1.2% of the administered dose. The level of hepatic copper should not be used to manage therapy since it does not differentiate between potentially toxic free copper and safely bound copper. In all treated patients, 24-hour urinary zinc levels may be a useful measure of compliance with the zinc acetate regimen. | 4.4 Special warnings and precautions for use Zinc acetate dihydrate is not recommended for the initial therapy of symptomatic patients because of its slow onset of action. Symptomatic patients must be initially treated with a chelating agent; once copper levels are below toxic thresholds and patients are clinically stable, maintenance treatment with Wilzin can be considered. Nevertheless, while awaiting zinc induced duodenal metallothionein production and consequential effective inhibition of copper absorption, zinc acetate dehydrate could be administered initially in symptomatic patients in combination with a chelating agent. Although rare, clinical deterioration may occur at the beginning of the treatment, as has also been reported with chelating agents. Whether this is related to mobilisation of copper stores or to natural history of the disease remains unclear. A change of therapy is recommended in this situation. Caution should be exercised when switching patients with portal hypertension from a chelating agent to Wilzin, when such patients are doing well and the treatment is tolerated. Two patients of a series of 16 died from hepatic decompensation and advanced portal hypertension after being changed from penicillamine to zinc therapy. Therapeutic monitoring The aim of the treatment is to maintain the plasma free copper (also known as non-ceruloplasmin plasma copper) below 250 microgram/l (normal: 100-150 microgram/l) and the urinary copper excretion below 125 microgram/24 h (normal: < 50 microgram/24 h). The non-ceruloplasmin plasma copper is calculated by subtracting the ceruloplasmin-bound copper from the total plasma copper, given that each milligram of ceruloplasmin contains 3 micrograms of copper. The urinary excretion of copper is an accurate reflection of body loading with excess copper only when patients are not on chelation therapy. Urinary copper levels are usually increased with chelation therapy such as penicillamine or trientine. The level of hepatic copper cannot be used to manage therapy since it does not differentiate between potentially toxic free copper and metallothionein bound copper. In treated patients, assays of urinary and/or plasma zinc may be a useful measure of treatment compliance. Values of urinary zinc above 2 mg/24 h and of plasma zinc above 1250 microgram/l generally indicate adequate compliance. Like with all anti-copper agents overtreatment carries the risk of copper deficiency, which is especially harmful for children and pregnant women since copper is required for proper growth and mental development. In these patient groups, urinary copper levels should be kept a little above the upper limit of normal or in the high normal range (i.e. 40 – 50 microgram/24 h). Laboratory follow-up including haematological surveillance and lipoproteins determination should also be performed in order to detect early manifestations of copper deficiency, such as anaemia and/or leukopenia resulting from bone marrow depression, and decrease in HDL cholesterol and HDL/total cholesterol ratio. As copper deficiency may also cause myeloneuropathy, physicians should be alert to sensory and motor symptoms and signs which may potentially indicate incipient neuropathy or myelopathy in patients treated with Wilzin. |
| Adverse Reactions | ADVERSE REACTIONS
Clinical experience with zinc acetate has been limited. The following adverse reactions have been reported in patients with Wilson’s disease on zinc therapy: a death following overdosage with zinc sulfate (See OVERDOSAGE) and a death in a patient with advanced liver disease and hemolytic crisis where zinc sulfate was used as initial treatment; gastric irritation; elevations of serum alkaline phosphatase, amylase and lipase lasting from weeks to months suggesting pancreatitis. The levels usually return to high normal within the first one or two years of zinc therapy. | 4.8 Undesirable effects Reported adverse reactions are listed below, by system organ class and by frequency. Frequencies are defined as: very common (≥ 1/10), common (≥ 1/100 to < 1/10), uncommon (≥ 1/1,000 to < 1/100), rare (≥ 1/10,000 to < 1/1,000), very rare (< 1/10,000), not known (cannot be estimated from the available data). Within each frequency grouping, undesirable effects are presented in order of decreasing seriousness. 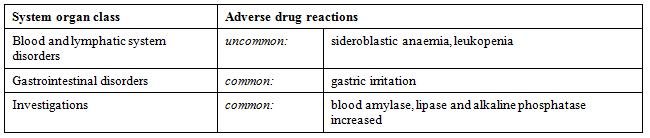 Anaemia may be micro-, normo- or macrocytic and is often associated with leukopenia. Bone marrow examination usually reveals characteristic "ringed sideroblasts" (i.e. developing red blood cells containing iron-engorged paranuclear mitochondria). They may be early manifestations of copper deficiency and may recover rapidly following reduction of zinc dosage. However, they must be distinguished from haemolytic anaemia which commonly occurs where there is elevated serum free copper in uncontrolled Wilson's disease. The most common undesirable effect is gastric irritation. This is usually worst with the first morning dose and disappears after the first days of treatment. Delaying the first dose to mid-morning or taking the dose with a little protein may usually relieve the symptoms. Elevations of serum alkaline phosphatase, amylase and lipase may occur after a few weeks of treatment, with levels usually returning to high normal within the first one or two years of treatment.
United Kingdom |
| Drug Interactions | Drug Interactions
Pharmacodynamic studies in Wilson’s disease patients failed to demonstrate drug interactions between zinc acetate (50 mg t.i.d.) and ascorbic acid (1 g daily), penicillamine (1 g daily), and trientine (1 g daily). Therefore, precautions for zinc acetate effects do not seem necessary when Wilson’s disease patients are taking vitamin C or approved chelating agents. However, no data are available to demonstrate that zinc acetate should be added to other drugs used for the treatment of Wilson’s disease patients or is safe. | 4.5 Interaction with other medicinal products and other forms of interaction
Other anti-copper agents Pharmacodynamic studies were conducted in Wilson's disease patients on the combination of Wilzin (50 mg three times daily) with ascorbic acid (1 g once daily), penicillamine (250 mg four times daily), and trientine (250 mg four times daily). They showed no significant overall effect on copper balance although mild interaction of zinc with chelators (penicillamine and trientine) could be detected with decreased faecal but increased urinary copper excretion as compared with zinc alone. This is probably due to some extent of complexion of zinc by the chelator, thus reducing the effect of both active substances. When switching a patient on chelating treatment to Wilzin for maintenance therapy, the chelating treatment should be maintained and co-administered for 2 to 3 weeks since this is the time it takes for the zinc treatment to induce maximum metallothionein induction and full blockade of copper absorption. The administration of the chelating treatment and Wilzin should be separated by at least 1 hour. Other medicinal products The absorption of zinc may be reduced by iron and calcium supplements, tetracyclines and phosphorus-containing compounds, while zinc may reduce the absorption of iron, tetracyclines, fluoroquinolones. Food Studies of the co-administration of zinc with food performed in healthy volunteers showed that the absorption of zinc was significantly delayed by many foods (including bread, hard boiled eggs, coffee and milk). Substances in food, especially phytates and fibres, bind zinc and prevent it from entering the intestinal cells. However, protein appears to interfere the least. |
| Special Populations | Nursing Mothers
Zinc does appear in breast milk and zinc-induced copper deficiency in the nursing baby may occur. Therefore, it is recommended that women on zinc therapy not nurse their babies. Pediatric Use Results of observations in a small number of patients in the two clinical trials suggest that pediatric patients aged 10 years and above can be adequately maintained at doses between 75 to 150 mg elemental zinc daily in divided doses. No patients below the age of 10 years have been studied. |
4.6 Pregnancy and lactation Pregnancy Data on a limited number of exposed pregnancies in patients with Wilson's disease give no indication of harmful effects of zinc on embryo/foetus and mother. Five miscarriages and 2 birth defects (microcephaly and correctable heart defect) were reported in 42 pregnancies. Animal studies conducted with different zinc salts do not indicate direct or indirect harmful effects with respect to pregnancy, embryonal/foetal development, parturition or postnatal development (see section 5.3). It is extremely important that pregnant Wilson's disease patients continue their therapy during pregnancy. Which treatment should be used, zinc or chelating agent should be decided by the physician. Dose adjustments to guarantee that the foetus will not become copper deficient must be done and close monitoring of the patient is mandatory (see section 4.4). Lactation Zinc is excreted in human breast milk and zinc-induced copper deficiency in the breast-fed baby may occur. Therefore, breast-feeding should be avoided during Wilzin therapy. |
| Abuse, Dependence, Overdosage | Drug Abuse and Dependence Zinc acetate has no potential for abuse, and it is not related pharmacologically or structurally to any other drug known to have abuse potential. OVERDOSAGE Acute oral overdosage with inorganic salts of zinc in humans is reported rarely. In the event of overdosage, the unabsorbed zinc salt should be removed from the stomach by lavage as quickly as possible. The plasma level of zinc should be measured, and heavy metal chelation therapy should be considered if the plasma level of zinc is elevated markedly (>1000 μg/dL). In addition, any signs or symptoms of toxicity should be treated as medically indicated. One fatality associated with overdosage of zinc sulfate has been reported. The death of this adult woman followed the accidental ingestion of approximately 28 g of zinc sulfate. Death occurred on the fifth day after ingestion and was attributed to renal failure. Hemorrhagic pancreatitis and hyperglycemic coma resulted from the overdose. The amount ingested was 500 mg/Kg of zinc sulfate, a value that is in the same order of magnitude as that found to be lethal in animals. | 4.9 Overdose Three cases of acute oral overdose with zinc salts (sulphate or gluconate) have been reported in the literature. Death occurred in a 35 year-old woman on the fifth day after ingestion of 6 g of zinc (40 times the proposed therapeutic dose) and was attributed to renal failure and haemorrhagic pancreatitis with hyperglycaemic coma. The same dose did not produce any symptoms except for vomiting in an adolescent who was treated by whole-bowel irrigation. Another adolescent who ingested 4 g of zinc had serum zinc level of about 50 mg/l 5 hours later and only experienced severe nausea, vomiting and dizziness. Treatment of overdose should be with gastric lavage or induced emesis as quickly as possible to remove unabsorbed zinc. Heavy metal chelation therapy should be considered if plasma zinc levels are markedly elevated (> 10 mg/l). |
| Carcinogenesis, Mutagenesis, Impairment of Fertility | Carcinogenesis, Mutagenesis, Impairment of Fertility
Zinc acetate has not been tested for its carcinogenic potential in long-term animal studies, for its mutagenic potential or for its effect on fertility in animals. However, testing with other salts of zinc (zinc oxide, zinc stearate, zinc sulfate) did not reveal a mutagenicity potential in in vitro Ames assays, and human embryonic lung cell chromosomal aberration assay, and in in vivo rat dominant lethal assay, and rat bone marrow cell chromosomal aberration assay. Other salts of zinc (zinc oxide, zinc chloride, zinc citrate, zinc maleate, zinc carbonate, zinc sulfate) and pure zinc dust at oral doses up to 326 mg/Kg/day (18 times the recommended human dose based on body surface area) were found to have no effect on fertility and reproductive performance of male and female rats. Pregnancy: Teratogenic Effects. Studies in pregnant women have not shown that zinc acetate or zinc sulfate increases the risk of fetal abnormalities if administered during all trimesters of pregnancy. If this drug is used during pregnancy, the possibility of fetal harm appears remote. Because studies cannot rule out the possibility of harm, however, zinc acetate should be used during pregnancy only if clearly needed. While zinc acetate should be used during pregnancy only if clearly needed, copper toxicosis can develop during pregnancy if anti-copper therapy is stopped. Oral teratology studies have been performed with zinc sulfate in pregnant rats at doses up to 42.5 mg/Kg/day (2 times the recommended human dose based on body surface area), mice at doses up to 30 mg/Kg/day (1 time the recommended human dose based on body surface area), rabbits at doses up to 60 mg/Kg/day (6 times the recommended human dose based on body surface area) and hamsters at doses up to 88 mg/Kg/day (5 times the recommended human dose based on body surface area) and have revealed no evidence of impaired fertility or harm to the fetus due to zinc sulfate. (See CLINICAL TRIALS). | 5.3 Preclinical safety data
Preclinical studies have been conducted with zinc acetate and with other zinc salts. Pharmacological and toxicological data available showed large similarities between zinc salts and among animal species. The oral LD50 is approximately 300 mg zinc/kg body weight (about 100 to 150 times the human therapeutic dose). Repeat-dose toxicity studies have established that the NOEL (No Observed Effect Level) is about 95 mg zinc/kg body weight (about 48 times the human therapeutic dose). The weight of evidence, from in vitro and in vivo tests, suggests that zinc has no clinically relevant genotoxic activity. Reproduction toxicology studies performed with different zinc salts showed no clinically relevant evidence of embryotoxicity, foetotoxicity or teratogenicity. No conventional carcinogenicity study has been conducted with zinc acetate dihydrate. |
| Company Information | Rx only. Rev. 7/2020 Distributed by: ©2020 Teva Pharmaceuticals USA, Inc. All rights reserved. | 7. Marketing authorisation holder
Recordati Rare Diseases Immeuble "Le Wilson" 70, avenue du Général de Gaulle F-92800 Puteaux France 8. Marketing authorisation number(s) EU/1/04/286/001 9. Date of first authorisation/renewal of the authorisation Date of first authorisation: 13 October 2004 Date of latest renewal: 13 October 2009 10. Date of revision of the text April 2019 Detailed information on this product is available on the website of the European Medicines Agency (EMEA) http://www.emea.europa.eu
Company Contact Details  |
| Clinical Data | CLINICAL TRIALS
In the single center United States trial, 60 patients with Wilson’s disease (31 male, 29 female) who had adequate detoxification of copper after initial chelation therapy were entered into a copper balance study of various dose regimens of zinc acetate. Patients were hospitalized to carefully control food and liquid intake. Food, urine and feces were analyzed for copper content, and copper balance was defined as the difference between copper intake and copper elimination/excretion over a 10-day period. A patient was considered in adequate copper balance if the result was less than +0.25 mg copper/day. Results for the groups in each dose regimen tested and for adequacy of individual results are provided in the following table. 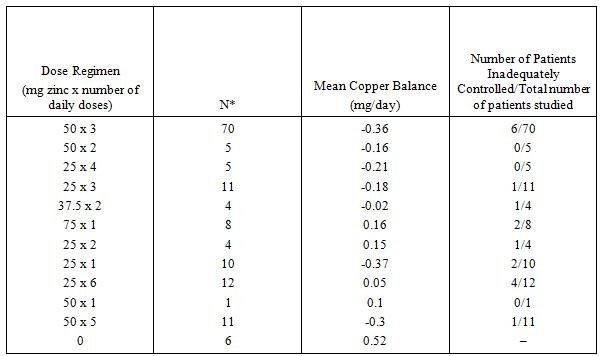 *N = number of copper balance studies. Some patients had more than one balance study, at different doses or at the same dose at widely separated intervals. While all zinc acetate regimens appeared better than no therapy, there was little experience with doses other than 50 mg t.i.d. Once daily dosing did not appear to give satisfactory control in many cases, and would be inadequate in patients with poor compliance. Based on the limited data available 25 mg t.i.d. was also thought to be an adequate dose regimen, and not shown to be inferior to 50 mg t.i.d. Dose related toxicity was not found in this study. Symptomatic Patients Initially Treated With a Chelating Drug Clinical parameters such as neuropsychiatric status including evaluation of speech, and liver function tests were followed as the patients continued therapy on an adequate zinc acetate dose regimen. One hundred and thirty-three patients were followed for up to 14 years. There was no deterioration of neuropsychiatric function including speech and biochemical liver function tests, including bilirubin, transaminases, alkaline phosphatase and lactic dehydrogenase. The liver function tests remained either within normal range or slightly above the upper limit of normal for up to 9 years of treatment. | There are no corresponding clinical data for WILZIN. |
| Clinical Data (continued) | Pre-symptomatic Patients
In this study 30 pre-symptomatic patients were followed for up to 10 years. Diagnosis of the pre-symptomatic Wilson’s disease was made on the basis of a liver copper value greater than 200 μg of copper per gram dry weight of tissue. Non-ceruloplasmin copper levels, 64Cu balance studies, and clinical parameters were assessed. No patient developed symptoms of Wilson’s disease in this cohort. Since the cloning and sequencing of the abnormal genes in Wilson’s disease patients, many mutations have been identified that may affect the rate of disease progression. No matched historical control has been compared to this experience, nor has another center replicated this experience. In a study in the Netherlands, using zinc sulfate, 27 patients were followed up to 29 years by mainly clinical parameters such as tremors, dysarthria, dystonia, ataxia and Kayser-Fleischer rings. No deterioration of the clinical status was observed. In some cases, Kayser-Fleischer rings disappeared and clinical signs and symptoms improved. Pregnant Patients Included in a continuing single center United States trial are 19 symptomatic and presymptomatic women who became pregnant and continued Galzin therapy. These women delivered 26 live birth babies. At the time of delivery, the duration of zinc acetate therapy had ranged from 0.7 to 13.7 years. At the time of delivery all patients were using zinc acetate. The zinc acetate dosage at the start of pregnancy ranged from 25 to 50 mg two to three times a day. Two patients were being treated with penicillamine at the start of pregnancy and were switched to zinc acetate during the second month of pregnancy. Urinary copper excretion was measured to monitor the copper status. Twenty-four hour urine excretion of copper indicated adequate control of copper levels in most patients before and during pregnancies. The results also indicated that during pregnancy, the mothers’ health was protected by zinc acetate therapy, and no adverse effects on liver or neurological functions were reported. Limited pregnancy outcome data indicates an incidence of miscarriages consistent with those in the general population. From this limited experience, the rate of birth defects is 7.7%, while that in the general population is (4%). (See PRECAUTIONS, Pregnancy). | |
| Patient Leaflet | There is no corresponding patient leaflet for GALZIN®. | PACKAGE LEAFLET: INFORMATION FOR THE USER
Wilzin 25 mg hard capsules / Wilzin 50 mg hard capsules - zinc |
Read all of this leaflet carefully before you start taking this medicine.
|
||
In this leaflet:
|
||
| 1. WHAT WILZIN IS AND WHAT IT IS USED FOR
Wilzin belongs to a group of medicines called Various Alimentary Tract and metabolism products. Wilzin is indicated in the treatment of Wilson’s disease, which is a rare inherited defect in copper excretion. Dietary copper, which cannot be properly eliminated, accumulates first in the liver, then in other organs such as the eyes and the brain. This potentially leads to liver damage and neurological disorders. Wilzin blocks the absorption of copper from the intestine thereby preventing its transfer into the blood and its further accumulation in the body. Unabsorbed copper is then eliminated in the stool. Wilson’s disease will persist during the entire lifetime of the patient and therefore the need for this treatment is life-long. |
||
| 2. BEFORE YOU TAKE WILZIN
Do not take Wilzin If you are allergic (hypersensitive) to zinc or any of the other ingredients of Wilzin. Take special care with Wilzin Wilzin is usually not recommended for initial therapy of patients with signs and symptoms of Wilson’s disease because of its slow onset of action. If you are currently treated with another anti-copper agent, for example, penicillamine, your doctor may add Wilzin before stopping the initial treatment. As with other anti-copper agents such as penicillamine, your symptoms may get worse after starting the treatment. In this case, you must inform your doctor. In order to follow up your condition and treatment your doctor will check your blood and urine on a regular basis. This is to ensure that you receive sufficient treatment. Monitoring may detect evidence of insufficient treatment (copper excess) or excessive treatment (copper deficiency), both of which can be harmful, particularly to growing children and pregnant women. You should tell your doctor if you experience unusual muscle weakness or abnormal feeling in your limbs as this may indicate excessive treatment. |
||
| Taking other medicines
Please tell your doctor or your pharmacist if you are taking or have recently taken any other medicines, including medicines obtained without a prescription. Please consult your doctor before taking any other medicines which may reduce the effectiveness of Wilzin, such as iron, calcium supplements, tetracyclines (antibiotics) or phosphorus. Conversely, the effectiveness of some medicines, such as iron, tetracyclines or fluoroquinolones (antibiotics), may be reduced by Wilzin. |
||
| Taking Wilzin with food and drink
Wilzin should be taken on an empty stomach, separated from mealtimes. Dietary fibres and some dairy products, in particular, delay the absorption of zinc salts. Some patients experience stomach upset after the morning dose. Please discuss the matter with your Wilson’s disease doctor if this affects you. This side effect may be reduced by postponing the first dose of the day until mid-morning (between breakfast and the midday meal). It may also be minimised by taking the first dose of Wilzin with a small amount of protein-containing food, such as meat (but not milk). |
||
| Pregnancy
Please consult your doctor if you plan to become pregnant. It is very important to continue anti-copper therapy during pregnancy. If you become pregnant during therapy with Wilzin, your doctor will decide which treatment and which dose is best in your situation. Breast-feeding Breast-feeding should be avoided if you are on Wilzin therapy. Please discuss with your doctor. Driving and using machines No studies of the effects on the ability to drive and use machines have been performed. Important information about some of the ingredients of Wilzin Wilzin 50 mg hard capsules contains sunset yellow FCF (E110) which may cause allergic reactions. |
||
| 3. HOW TO TAKE WILZIN
Always take Wilzin exactly as your doctor has told you. You should check with your doctor or pharmacist if you are not sure. For the different dose regimens Wilzin is available in hard capsules of 25 mg or 50 mg. • For adults: The usual dose is 1 hard capsule of Wilzin 50 mg (or 2 hard capsules of Wilzin 25 mg) three times daily with a maximum dose of 1 hard capsule of Wilzin 50 mg (or 2 hard capsules of Wilzin 25 mg) five times daily. • For children and adolescents: The usual dose is: -from 1 to 6 years: 1 hard capsule of Wilzin 25 mg twice daily -from 6 to 16 years if bodyweight under 57 kg: 1 hard capsule of Wilzin 25 mg three times daily - from 16 years or if bodyweight above 57 kg: 2 hard capsules of Wilzin 25 mg or 1 hard capsule of Wilzin 50 mg three times daily. Always take Wilzin on an empty stomach, at least one hour before or 2-3 hours after meals. If the morning dose is not well tolerated (see section 4) it is possible to delay it to mid-morning, between breakfast and lunch. It is also possible to take Wilzin with a little protein, such as meat. If you have been prescribed Wilzin with another anti-copper agent, such as penicillamine, keep an interval of at least 1 hour between the two medicines. To administer Wilzin to children who are unable to swallow capsules, open the capsule and mix the powder with a little water (possibly flavoured with sugar or syrup). If you take more Wilzin than you should: If you take more Wilzin than prescribed, you may experience nausea, vomiting and dizziness. In this case you must ask your doctor for advice. If you forget to take Wilzin: Do not take a double dose to make up for a forgotten individual dose. If you have any further questions on the use of this medicine, ask your doctor. |
||
| 4. POSSIBLE SIDE EFFECTS
Like all medicines, Wilzin can cause side effects, although not everybody gets them. These side effects may occur with certain frequencies, which are defined as follows: • very common: affects more than 1 user in 10 • common: affects 1 to 10 users in 100 • uncommon: affects 1 to 10 users in 1,000 • rare: affects 1 to 10 users in 10,000 • very rare: affects less than 1 user in 10,000 • not known: frequency cannot be estimated from the available data. Common: • After Wilzin intake, gastric irritation may occur, especially at the beginning of treatment. • Changes in blood tests have been reported, including an increase in some liver and pancreatic enzymes. Uncommon: • A decrease in blood red and white cells may occur. Reporting of side effects If you get any side effects, talk to your doctor or, pharmacist. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via the national reporting system listed in Appendix. By reporting side effects you can help provide more information on the safety of this medicine. |
||
| 5. HOW TO STORE WILZIN
• Keep out of the reach and sight of children. • Do not use Wilzin after the expiry date stated on the bottle and the carton, after EXP. The expiry date refers to the last day of that month. • Do not store above 25°C. Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. These measures will help to protect the environment. |
||
| 6. FURTHER INFORMATION
What Wilzin contains The active substance is zinc. Each hard capsule contains 25 mg of zinc (corresponding to 83.92 mg of zinc acetate dihydrate) or 50 mg of zinc (corresponding to 167.84 mg of zinc acetate dihydrate). The other ingredients are maize starch and magnesium stearate. The capsule shell contains gelatin, titanium dioxide (E171) and either brilliant blue FCF (E133) for Wilzin 25 mg, or sunset yellow FCF (E110) for Wilzin 50 mg. The printing ink contains black iron oxide (E172) and shellac. What Wilzin looks like and contents of the pack Wilzin 25 mg is an aqua blue hard capsule imprinted «93-376”. Wilzin 50 mg is an orange opaque hard capsule imprinted “93-377”. It is available in packs of 250 hard capsules in a polyethylene bottle closed by a polypropylene and polyethylene closure. The bottle also contains a cotton filler. |
||
| Marketing Authorisation Holder and Manufacturer
Recordati Rare Diseases For any information about this medicine, please contact the local representative of the Marketing Authorisation Holder. This leaflet was last approved in 04/2019 Detailed information on this medicine is available on the European Medicines Agency (EMA) web site: http://www.ema.europa.eu. There are also links to other websites about rare diseases and treatments. |

APPENDIX 3
Instructions on How to Obtain WILZIN® as Alternate Supply to GALZIN®
In coordination with the U. S. Food and Drug Administration (FDA), Teva is temporarily distributing WILZIN® (zinc acetate dihydrate) capsules as an alternate supply to GALZIN® (zinc acetate) capsules in the U.S. to increase the availability of this drug following a supply disruption of GALZIN®. To ensure uninterrupted access to patients in need of this therapy, Teva will provide WILZIN® at no cost to patients until GALZIN® brand is available for prescription again in the United States. WILZIN® will be shipped to a pharmacy of the patient’s choice upon completion and verification of the information requested in the form below by a healthcare provider. A maximum 60 day supply of WILZIN® may be requested to ensure there is adequate allocation available for as many patients as possible. Please reference the following instructions for requesting WILZIN® supply.
How do I access WILZIN®?
To access the WILZIN® product from Teva, call the main Teva toll-free number: 1-888-838-2872 and press 3 to speak with Patricia Prezioso, Customer Service Supervisor. In the event Patricia is unavailable, James Chiccone will be available to assist. Teva representatives are aware of the critical nature of supplying WILZIN® to patients, so if a voice mailbox is reached, please leave a message and your call will be promptly returned. Accordingly, Teva will provide WILZIN® as a “drop-shipment,” which means you will need to provide the required information in the form below.
Teva anticipates the time needed to verify the information in the completed form is approximately 24 hours. Teva will ship medication to the pharmacy at no cost to the patient and patient’s pharmacy to ensure ease of access.

WILZIN® Supply Request Form
Teva Customer Service:
TevaCS@TevaPharm.com | 888-838-2872, Option 3
|
NDC: 57844-0376-25 Description: WILZIN 25 mg (zinc acetate dihydrate) capsules (250 capsules/bottle) |
|
Number of bottles requested to treat patient for 60 days: |
| Prescribing Physician Name: |
| Prescribing Physician Phone: |
|
Name of Pharmacy’s Wholesaler: |
| Pharmacy Name: | ||
| Address 1: | ||
| Address 2: | ||
| City: | State: | Zip: |
| Contact Name: | Contact Phone: | |
| State provided Board of Pharmacy License Number: _____________________________ (Please provide a hard copy of the pharmacy license to TevaCS@tevapharm.com) |
Once your request is reviewed and approved, the approval will be sent back to you. You would then forward that approval to your wholesaler for them to place the drop-shipment order on your behalf.
Teva Pharmaceutical USA, Inc.
400 Interpace Pkwy, Parsippany, NJ 07054
PATIENT PACKAGE INSERT
PACKAGE LEAFLET: INFORMATION FOR THE USER
Wilzin 25 mg hard capsules / Wilzin 50 mg hard capsules - zinc
Read all of this leaflet carefully before you start taking this medicine.
- Keep this leaflet. You may need to read it again.
- If you have any further questions, please ask your doctor or pharmacist.
- This medicine has been prescribed for you personally. Do not pass it on to others. It may harm them, even if their symptoms are the same as yours.
- If any of the side effects gets serious, or if you notice any side effects not listed in this leaflet, please tell your doctor or pharmacist.
In this leaflet:
- What Wilzin is and what it is used for
- Before you take Wilzin
- How to take Wilzin
- Possible side effects
- How to store Wilzin
- Further information
1. WHAT WILZIN IS AND WHAT IT IS USED FOR
Wilzin belongs to a group of medicines called Various Alimentary Tract and metabolism products.
Wilzin is indicated in the treatment of Wilson’s disease, which is a rare inherited defect in copper excretion. Dietary copper, which cannot be properly eliminated, accumulates first in the liver, then in other organs such as the eyes and the brain. This potentially leads to liver damage and neurological disorders.
Wilzin blocks the absorption of copper from the intestine thereby preventing its transfer into the blood and its further accumulation in the body. Unabsorbed copper is then eliminated in the stool.
Wilson’s disease will persist during the entire lifetime of the patient and therefore the need for this treatment is life-long.
2. BEFORE YOU TAKE WILZIN
Do not take Wilzin
If you are allergic (hypersensitive) to zinc or any of the other ingredients of Wilzin.
Take special care with Wilzin
Wilzin is usually not recommended for initial therapy of patients with signs and symptoms of Wilson’s disease because of its slow onset of action.
If you are currently treated with another anti-copper agent, for example, penicillamine, your doctor may add Wilzin before stopping the initial treatment.
As with other anti-copper agents such as penicillamine, your symptoms may get worse after starting the treatment. In this case, you must inform your doctor.
In order to follow up your condition and treatment your doctor will check your blood and urine on a regular basis. This is to ensure that you receive sufficient treatment. Monitoring may detect evidence of insufficient treatment (copper excess) or excessive treatment (copper deficiency), both of which can be harmful, particularly to growing children and pregnant women.
You should tell your doctor if you experience unusual muscle weakness or abnormal feeling in your limbs as this may indicate excessive treatment.
Taking other medicines
Please tell your doctor or your pharmacist if you are taking or have recently taken any other medicines, including medicines obtained without a prescription.
Please consult your doctor before taking any other medicines which may reduce the effectiveness of Wilzin, such as iron, calcium supplements, tetracyclines (antibiotics) or phosphorus. Conversely, the effectiveness of some medicines, such as iron, tetracyclines or fluoroquinolones (antibiotics), may be reduced by Wilzin.
Taking Wilzin with food and drink
Wilzin should be taken on an empty stomach, separated from mealtimes. Dietary fibres and some dairy products, in particular, delay the absorption of zinc salts. Some patients experience stomach upset after the morning dose. Please discuss the matter with your Wilson’s disease doctor if this affects you.
This side effect may be reduced by postponing the first dose of the day until mid-morning (between breakfast and the midday meal). It may also be minimised by taking the first dose of Wilzin with a small amount of protein-containing food, such as meat (but not milk).
Pregnancy
Please consult your doctor if you plan to become pregnant. It is very important to continue anti-copper therapy during pregnancy.
If you become pregnant during therapy with Wilzin, your doctor will decide which treatment and which dose is best in your situation.
Breast-feeding
Breast-feeding should be avoided if you are on Wilzin therapy. Please discuss with your doctor.
Driving and using machines
No studies of the effects on the ability to drive and use machines have been performed.
Important information about some of the ingredients of Wilzin
Wilzin 50 mg hard capsules contains sunset yellow FCF (E110) which may cause allergic reactions.
3. HOW TO TAKE WILZIN
Always take Wilzin exactly as your doctor has told you. You should check with your doctor or pharmacist if you are not sure. For the different dose regimens Wilzin is available in hard capsules of 25 mg or 50 mg.
• For adults:
The usual dose is 1 hard capsule of Wilzin 50 mg (or 2 hard capsules of Wilzin 25 mg) three times daily with a maximum dose of 1 hard capsule of Wilzin 50 mg (or 2 hard capsules of Wilzin 25 mg) five times daily.
• For children and adolescents:
The usual dose is:
-from 1 to 6 years: 1 hard capsule of Wilzin 25 mg twice daily
-from 6 to 16 years if bodyweight under 57 kg: 1 hard capsule of Wilzin 25 mg three times daily
- from 16 years or if bodyweight above 57 kg: 2 hard capsules of Wilzin 25 mg or 1 hard capsule of Wilzin 50 mg three times daily.
Always take Wilzin on an empty stomach, at least one hour before or 2-3 hours after meals.
If the morning dose is not well tolerated (see section 4) it is possible to delay it to mid-morning, between breakfast and lunch. It is also possible to take Wilzin with a little protein, such as meat.
If you have been prescribed Wilzin with another anti-copper agent, such as penicillamine, keep an interval of at least 1 hour between the two medicines.
To administer Wilzin to children who are unable to swallow capsules, open the capsule and mix the powder with a little water (possibly flavoured with sugar or syrup).
If you take more Wilzin than you should:
If you take more Wilzin than prescribed, you may experience nausea, vomiting and dizziness. In this case you must ask your doctor for advice.
If you forget to take Wilzin:
Do not take a double dose to make up for a forgotten individual dose. If you have any further questions on the use of this medicine, ask your doctor.
4. POSSIBLE SIDE EFFECTS
Like all medicines, Wilzin can cause side effects, although not everybody gets them.
These side effects may occur with certain frequencies, which are defined as follows:
• very common: affects more than 1 user in 10
• common: affects 1 to 10 users in 100
• uncommon: affects 1 to 10 users in 1,000
• rare: affects 1 to 10 users in 10,000
• very rare: affects less than 1 user in 10,000
• not known: frequency cannot be estimated from the available data.
Common:
• After Wilzin intake, gastric irritation may occur, especially at the beginning of treatment.
• Changes in blood tests have been reported, including an increase in some liver and pancreatic enzymes.
Uncommon:
• A decrease in blood red and white cells may occur.
Reporting of side effects
If you get any side effects, talk to your doctor or, pharmacist. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via the national reporting system listed in Appendix. By reporting side effects you can help provide more information on the safety of this medicine.
5. HOW TO STORE WILZIN
• Keep out of the reach and sight of children.
• Do not use Wilzin after the expiry date stated on the bottle and the carton, after EXP. The expiry date refers to the last day of that month.
• Do not store above 25°C.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. These measures will help to protect the environment.
6. FURTHER INFORMATION
What Wilzin contains
The active substance is zinc. Each hard capsule contains 25 mg of zinc (corresponding to 83.92 mg of zinc acetate dihydrate) or 50 mg of zinc (corresponding to 167.84 mg of zinc acetate dihydrate). The other ingredients are maize starch and magnesium stearate. The capsule shell contains gelatin, titanium dioxide (E171) and either brilliant blue FCF (E133) for Wilzin 25 mg, or sunset yellow FCF (E110) for Wilzin 50 mg. The printing ink contains black iron oxide (E172) and shellac.
What Wilzin looks like and contents of the pack
Wilzin 25 mg is an aqua blue hard capsule imprinted «93-376”. Wilzin 50 mg is an orange opaque hard capsule imprinted “93-377”.
It is available in packs of 250 hard capsules in a polyethylene bottle closed by a polypropylene and polyethylene closure. The bottle also contains a cotton filler.
Marketing Authorisation Holder and Manufacturer
Recordati Rare Diseases
Immeuble “Le Wilson”
70 avenue du Général de Gaulle
F-92800 Puteaux -France
For any information about this medicine, please contact the local representative of the Marketing Authorisation Holder.
This leaflet was last approved in 04/2019
Detailed information on this medicine is available on the European Medicines Agency (EMA) web site: http://www.ema.europa.eu. There are also links to other websites about rare diseases and treatments.
| WILZIN
zinc acetate dihydrate capsule |
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
 |
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
| Labeler - Teva Pharmaceuticals USA, Inc. (001627975) |
Frequently asked questions
More about Wilzin (zinc acetate)
- Check interactions
- Compare alternatives
- Drug images
- Side effects
- Dosage information
- During pregnancy
- Drug class: minerals and electrolytes


