Equate Acid Controller Maximum Strength: Package Insert / Prescribing Info
Package insert / product label
Generic name: famotidine
Dosage form: tablet
Indications and Usage for Equate Acid Controller Maximum Strength
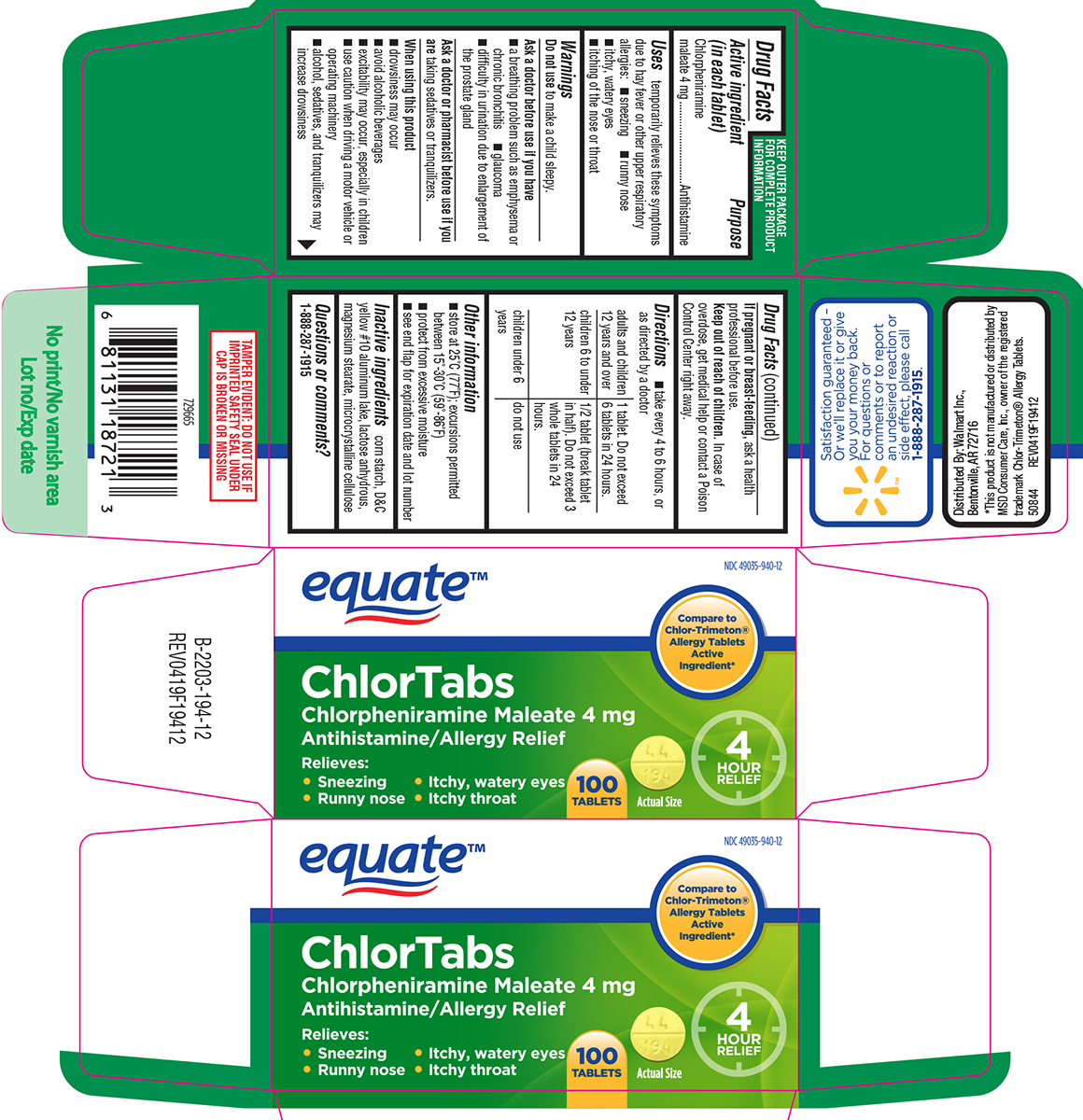
temporarily relieves these symptoms due to hay fever or other upper respiratory allergies:
- runny nose
- sneezing
- itchy, watery eyes
- itching of the nose or throat
Warnings
Ask a doctor before use if you have
- a breathing problem such as emphysema or chronic bronchitis
- glaucoma
- difficulty in urination due to enlargement of the prostate gland
Equate Acid Controller Maximum Strength Dosage and Administration
- take every 4 to 6 hours, or as directed by a doctor
| adults and children 12 years and over | 1 tablet. Do not exceed 6 tablets in 24 hours. |
| children 6 to under 12 years | 1/2 tablet (break tablet in half). Do not exceed 3 whole tablets in 24 hours. |
| children under 6 years | do not use |
Storage and Handling
- store at 25ºC (77ºF); excursions permitted between 15º-30ºC (59º-86ºF)
- protect from excessive moisture
- see end flap for expiration date and lot number
Inactive ingredients
corn starch, D&C yellow #10 aluminum lake, lactose anhydrous, magnesium stearate, microcrystalline cellulose
Principal Display Panel
equate™
NDC 49035-940-12
Compare to
Chlor-Trimeton®
Allergy Tablets
Active
Ingredient*
ChlorTabs
Chlorpheniramine Maleate 4 mg
Antihistamine / Allergy Relief
Relieves:
• Sneezing • Itchy, watery eyes
• Runny nose • Itchy throat
100
TABLETS
Actual Size
4
HOUR
RELIEF
TAMPER EVIDENT: DO NOT USE IF
IMPRINTED SAFETY SEAL UNDER
CAP IS BROKEN OR MISSING
Satisfaction guaranteed -
Or we'll replace it or give
you your money back.
For questions or
comments or to report
an undesired reaction or
side effect, please call
1-888-287-1915.
Distributed by: Walmart Inc.,
Bentonville, AR 72716
*This product is not manufactured or distributed by
MSD Consumer Care, Inc., owner of the registered
trademark Chlor-Trimeton® Allergy Tablets.
50844 REV0419F19412
Equate 44-194
| CHLORTABS
chlorpheniramine maleate tablet |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Wal-Mart Stores Inc (051957769) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| LNK International, Inc. | 038154464 | pack(49035-940) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| LNK International, Inc. | 832867837 | pack(49035-940) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| LNK International, Inc. | 832867894 | manufacture(49035-940) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| LNK International, Inc. | 868734088 | pack(49035-940) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| LNK International, Inc. | 967626305 | pack(49035-940) | |
