CitraNatal 90 DHA: Package Insert / Prescribing Info
Package insert / product label
Generic name: vitamin C, calcium, iron, vitamin D3, vitamin E, thiamin, riboflavin, niacinamide, vitamin B6, folic acid, iodine, zinc, copper, docusate sodium
Dosage form: tablet
Drug classes: Iron products, Vitamin and mineral combinations
Medically reviewed by Drugs.com. Last updated on Jan 14, 2024.
On This Page
WARNING:Accidental overdose of iron-containingproducts is a leading cause of fatal poisoning in children under 6. KEEP THIS PRODUCT OUT OF THE REACH OF CHILDREN. In case of accidental overdose, call a doctor or poison control center immediately.
DESCRIPTION
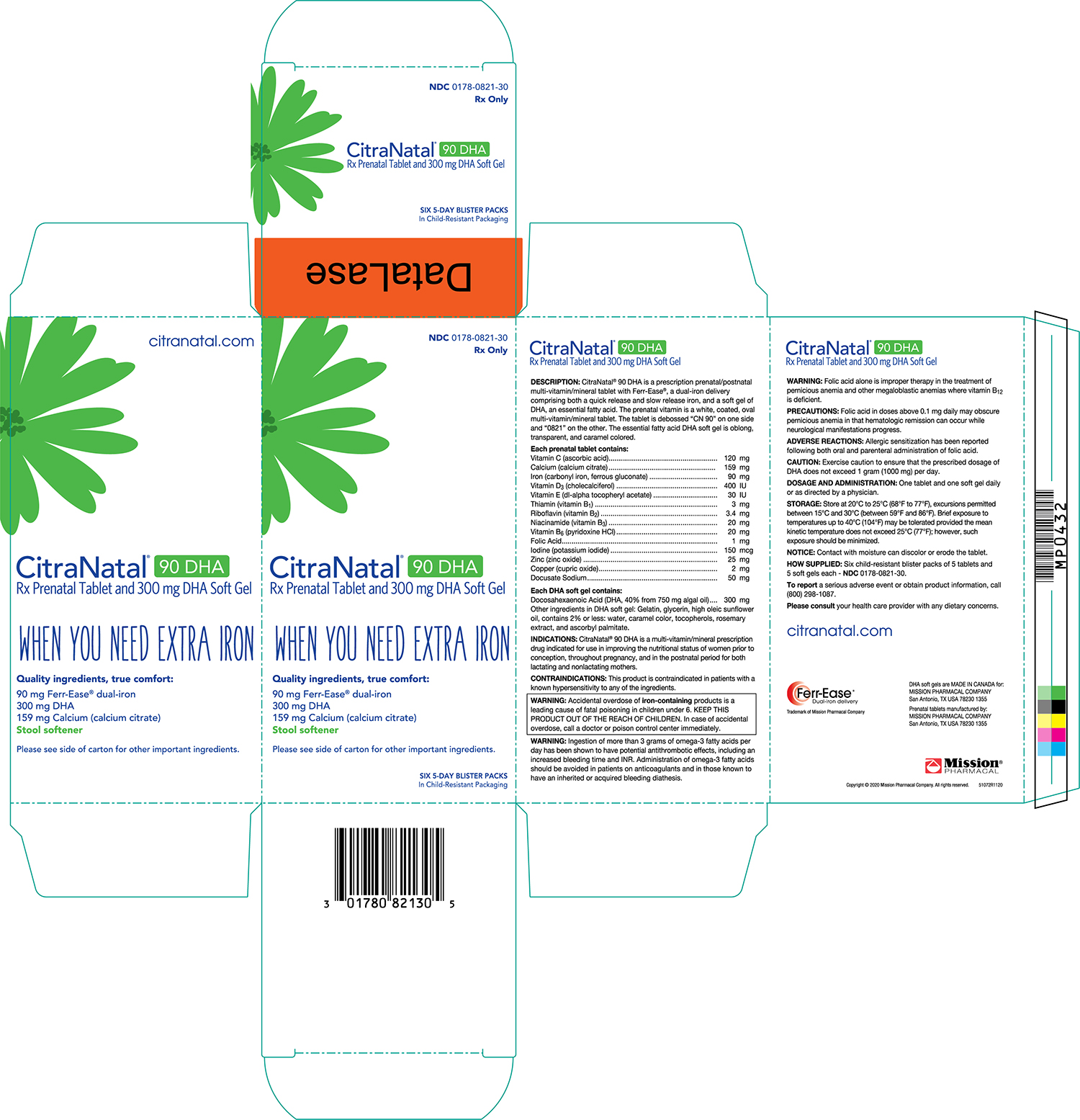
CitraNatal ®90 DHA is a prescription prenatal/postnatal multivitamin/mineral tablet with Ferr-Ease ®, a patented dual-iron delivery comprising both a quick release and slow release iron, and a capsule of an essential fatty acid. The prenatal vitamin is a white, oval multivitamin/mineral tablet. The tablet is debossed “CN 90” on one side and “0821” on the other. The essential fatty acid DHA capsule is caramel colored and contains a light yellow to orange semi-solid mixture.
| Vitamin C (Ascorbic acid) | 120 mg |
| Calcium (Calcium citrate) | 159 mg |
| Iron (Carbonyl iron, ferrous gluconate) | 90 mg |
| Vitamin D3 (Cholecalciferol) | 400 IU |
| Vitamin E (dl-alpha tocopheryl acetate) | 30 IU |
| Thiamin (Vitamin B1) | 3 mg |
| Riboflavin (Vitamin B2) | 3.4 mg |
| Niacinamide (Vitamin B3) | 20 mg |
| Vitamin B6 (Pyridoxine HCl) | 20 mg |
| Folic Acid | 1 mg |
| Iodine (Potassium iodide) | 150 mcg |
| Zinc (Zinc oxide) | 25 mg |
| Copper (Cupric oxide) | 2 mg |
| Docusate Sodium | 50 mg |
| Docosahexaenoic | |
| Acid(DHA, 40% from 750 mg Algal Oil) | 300 mg |
| Eicosapentaenoic Acid (EPA) | Not more than 0.750 mg |
Other ingredients in DHA gelatin capsule: Gelatin, glycerin, sunflower oil, water, caramel color, tocopherols, rosemary extract, sunflower lecithin, ascorbyl palmitate.
Indications and Usage for CitraNatal 90 DHA
CitraNatal ®90 DHA is a multivitamin/mineral prescription drug indicated for use in improving the nutritional status of women prior to conception, throughout pregnancy, and in the postnatal period for both lactating and nonlactating mothers.
Contraindications
This product is contraindicated in patients with a known hypersensitivity to any of the ingredients.
Warnings
Ingestion of more than 3 grams of omega-3 fatty acids per day has been shown to have potential antithrombotic effects, including an increased bleeding time and INR. Administration of omega-3 fatty acids should be avoided in patients on anticoagulants and in those known to have an inherited or acquired bleeding diathesis.
WARNING:Folic acid alone is improper therapy in the treatment of pernicious anemia and other megaloblastic anemias where vitamin B 12is deficient.
Precautions
Folic acid in doses above 0.1 mg daily may obscure pernicious anemia in that hematologic remission can occur while neurological manifestations progress.
Adverse Reactions/Side Effects
Allergic sensitization has been reported following both oral and parenteral administration of folic acid.
CitraNatal 90 DHA Dosage and Administration
One tablet and one capsule daily or as directed by a physician.
Storage and Handling
NOTICE:Contact with moisture can discolor or erode the tablet.
HOW SUPPLIED
Six child-resistant blister packs of 5 tablets and 5 capsules each - NDC0178-0821-30
To reporta serious adverse event or obtain product information, call (210) 696-8400.
Please consultyour health care provider with any dietary concerns.
51072R1120
DHA capsules manufactured for:
MISSION PHARMACAL COMPANY
San Antonio, TX USA 78230 1355
Prenatal tablets manufactured by:
MISSION PHARMACAL COMPANY
San Antonio, TX USA 78230 1355
Ferr-Ease™
Dual-iron delivery
Trademark of Mission Pharmacal Company
| CITRANATAL 90 DHA
vitamin c, calcium, iron, vitamin d3, vitamin e, thiamin, riboflavin, niacinamide, vitamin b6, folic acid, iodine, zinc, copper, docusate sodium tablet |
||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - Mission Pharmacal Company (008117095) |
| Registrant - Mission Pharmacal Company (927726893) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Mission Pharmacal Company | 927726893 | manufacture(0178-0821) | |
More about CitraNatal 90 DHA (multivitamin, prenatal)
- Check interactions
- Compare alternatives
- Reviews (15)
- Side effects
- Dosage information
- Drug class: iron products
Patient resources
Professional resources
Other brands
Prenatal 19, Prenatal Plus Iron, Prenatal Plus, PreNexa, ... +35 more