Cefazolin and Dextrose: Package Insert / Prescribing Info
Package insert / product label
Generic name: cefazolin sodium
Dosage form: injection
Drug class: First generation cephalosporins
J Code (medical billing code): J0690 (Up to 500 mg, injection)
Medically reviewed by Drugs.com. Last updated on Nov 20, 2024.
On This Page
- Indications and Usage
- Dosage and Administration
- Dosage Forms and Strengths
- Contraindications
- Warnings and Precautions
- Adverse Reactions/Side Effects
- Drug Interactions
- Use In Specific Populations
- Overdosage
- Description
- Clinical Pharmacology
- Nonclinical Toxicology
- References
- How Supplied/Storage and Handling
- Patient Counseling Information
Highlights of Prescribing Information
CEFAZOLIN FOR INJECTION AND DEXTROSE INJECTION, for intravenous use
Initial U.S. Approval: 1973
Recent Major Changes
Indications and Usage for Cefazolin and Dextrose
Cefazolin for Injection and Dextrose Injection is a cephalosporin antibacterial indicated for:
- Treatment of the following infections caused by susceptible isolates of the designated microorganisms in adult and pediatric patients for whom appropriate dosing with this formulation can be achieved: (1)
- Respiratory tract infections (1.1)
- Urinary tract infections (1.2)
- Skin and skin structure infections (1.3)
- Biliary tract infections (1.4)
- Bone and joint infections (1.5)
- Genital infections (1.6)
- Septicemia (1.7)
- Endocarditis (1.8)
- Perioperative prophylaxis in adults and pediatric patients aged
10 to 17 years old for whom appropriate dosing with this formulation can be achieved (1.9)
To reduce the development of drug-resistant bacteria and maintain the effectiveness of Cefazolin for Injection and Dextrose Injection and other antibacterial drugs, Cefazolin for Injection and Dextrose Injection should be used only to treat or prevent infections that are proven or strongly suspected to be caused by bacteria. (1.10).
Cefazolin and Dextrose Dosage and Administration
If a dose of Cefazolin for Injection and Dextrose Injection is required that does not equal 1 gram, 2 grams or 3 grams, this product is not recommended for use and an alternative formulation of cefazolin should be considered. (2.1)
For intravenous use only administered over approximately 30 minutes. (2.1)
|
||
| Recommended Dosing Schedule in Adult Patients with CLcr Greater Than or Equal To 55 mL/min. (2.1, 2.2 and 2.3) | ||
| Site and Type of Infection | Dose | Frequency |
| Moderate to severe infections | 500 milligram (mg) to 1 gram | every 6 to 8 hours |
| Mild infections caused by susceptible gram-positive cocci | 250 mg to 500 mg | every 8 hours |
| Acute, uncomplicated urinary tract infections | 1 gram | every 12 hours |
| Pneumococcal pneumonia | 500 mg | every 12 hours |
| Severe, life-threatening infections (e.g., endocarditis, septicemia)* | 1 gram to 1.5 grams | every 6 hours |
| Perioperative prophylaxis | less than 120 kg: 1 gram to 2 grams | 1/2 to 1 hour prior to start of surgery |
| greater than or equal to 120 kg: 3 grams |
||
| 500 mg to 1 gram | additional dose during lengthy operative procedures |
|
| 500 mg to 1 gram | every 6 to 8 hours for 24 hours postoperatively | |
|
||
| Recommended Dosing Schedule in Pediatric Patients with CLcr Greater than or Equal to 70 mL/min. (2.1, 2.2, and 2.3) | ||
| Site and Type of Infection | Dose | Frequency |
| Moderate to severe infections* | 25 to 50 mg per kilogram (kg) | divided into 3 or 4 equal doses |
| Severe infections* | May increase to 100 mg per kg | divided into 3 or 4 equal doses |
|
Perioperative prophylaxis (10 to 17 years old) | less than 50 kg: 1 gram | 1/2 to 1 hour prior to start of surgery |
| greater than or equal to 50 kg: 2 grams | ||
| 500 mg to 1 gram | Additional dose during lengthy operative procedures |
|
| 500 mg to 1 gram | every 6 to 8 hours for 24 hours postoperatively | |
- Dosage adjustment is required for adult patients with CLcr that is less than 55 mL/min and pediatric patients with CLcr that is less than 70 mL/min. (2.4 and 8.6)
- See full prescribing information for preparation and administration instructions. (2.5)
Dosage Forms and Strengths
Contraindications
- Hypersensitivity to cefazolin or other cephalosporin class antibacterial drugs, penicillins, or other beta-lactams (4.1)
Warnings and Precautions
- Hypersensitivity reactions: Cross-hypersensitivity may occur in up to 10% of patients with a history of penicillin allergy. If an allergic reaction occurs, discontinue the drug. (5.1)
- Clostridioides difficile-associated Diarrhea (CDAD): May range from mild diarrhea to fatal colitis. Evaluate if diarrhea occurs. (5.3)
- Prothrombin Activity: May be associated with a fall in prothrombin activity. Prothrombin time should be monitored in patients at risk and exogenous vitamin K administered as indicated (5.5)
Adverse Reactions/Side Effects
- Adult and Pediatric Patients: Most common adverse reactions: gastrointestinal (nausea, vomiting, diarrhea), and allergic reactions (anaphylaxis, urticaria, skin rash). (6)
- Pediatric Patients with Perioperative Prophylaxis: The most frequently reported adverse reactions (incidence ≥ 5%) were nausea, infusion site pain, and headache. (6)
To report SUSPECTED ADVERSE REACTIONS, contact B. Braun Medical Inc. at 1-800-854-6851 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Drug Interactions
- Probenecid: The renal excretion of cefazolin is inhibited by probenecid. Co-administration of probenecid with cefazolin for injection is not recommended. (7)
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 9/2024
Full Prescribing Information
1. Indications and Usage for Cefazolin and Dextrose
1.1 Respiratory Tract Infections
Cefazolin for Injection and Dextrose Injection is indicated for the treatment of respiratory tract infections due to Streptococcus pneumoniae, Staphylococcus aureus and Streptococcus pyogenes in adults and pediatric patients for whom appropriate dosing with this formulation can be achieved [see Dosage and Administration (2.1, 2.2, 2.4, 2.5) and Use in Specific Populations (8.4)].
Limitations of Use
Injectable benzathine penicillin is considered the drug of choice in treatment and prevention of streptococcal infections, including the prophylaxis of rheumatic fever.
Cefazolin for Injection and Dextrose Injection is indicated for the eradication of streptococci from the nasopharynx; however, data establishing the efficacy of cefazolin in the subsequent prevention of rheumatic fever are not available.
1.2 Urinary Tract Infections
Cefazolin for Injection and Dextrose Injection is indicated for the treatment of urinary tract infections due to Escherichia coli, and Proteus mirabilis in adults and pediatric patients for whom appropriate dosing with this formulation can be achieved [see Dosage and Administration (2.1, 2.2, 2.4, 2.5) and Use in Specific Populations (8.4)].
1.3 Skin and Skin Structure Infections
Cefazolin for Injection and Dextrose Injection is indicated for the treatment of skin and skin structure infections due to S. aureus, S. pyogenes, and Streptococcus agalactiae in adults and pediatric patients for whom appropriate dosing with this formulation can be achieved [see Dosage and Administration (2.1, 2.2, 2.4, 2.5) and Use in Specific Populations (8.4)].
1.4 Biliary Tract Infections
Cefazolin for Injection and Dextrose Injection is indicated for the treatment of biliary infections due to E. coli, various isolates of streptococci, P. mirabilis, and S. aureus in adults and pediatric patients for whom appropriate dosing with this formulation can be achieved [see Dosage and Administration (2.1, 2.2, 2.4, 2.5) and Use in Specific Populations (8.4)].
1.5 Bone and Joint Infections
Cefazolin for Injection and Dextrose Injection is indicated for the treatment of bone and joint infections due to S. aureus in adults and pediatric patients for whom appropriate dosing with this formulation can be achieved [see Dosage and Administration (2.1, 2.2, 2.4, 2.5) and Use in Specific Populations (8.4)].
1.6 Genital Infections
Cefazolin for Injection and Dextrose Injection is indicated for the treatment of genital infections due to E. coli, and P. mirabilis in adults and pediatric patients for whom appropriate dosing with this formulation can be achieved [see Dosage and Administration (2.1, 2.2, 2.4, 2.5) and Use in Specific Populations (8.4)].
1.7 Septicemia
Cefazolin for Injection and Dextrose Injection is indicated for the treatment of septicemia due to S. pneumoniae, S. aureus, P. mirabilis, and E. coli in adults and pediatric patients for whom appropriate dosing with this formulation can be achieved [see Dosage and Administration (2.1, 2.2, 2.4, 2.5) and Use in Specific Populations (8.4)].
1.8 Endocarditis
Cefazolin for Injection and Dextrose Injection is indicated for the treatment of endocarditis due to S. aureus and S. pyogenes in adults and pediatric patients for whom appropriate dosing with this formulation can be achieved [see Dosage and Administration (2.1, 2.2, 2.4, 2.5) and Use in Specific Populations (8.4)].
1.9 Perioperative Prophylaxis
Cefazolin for Injection and Dextrose Injection is indicated for perioperative prophylaxis in adults and pediatric patients aged 10 to 17 years old for whom appropriate dosing with this formulation can be achieved [see Dosage and Administration (2.1, 2.3, 2.4, 2.5) and Use in Specific Populations (8.4)].
The perioperative use of Cefazolin for Injection and Dextrose Injection is indicated in adult and pediatric (aged 10 to 17 years old) surgical patients in whom infection at the operative site would present a serious risk (e.g., during open-heart surgery and prosthetic arthroplasty).
The prophylactic administration of Cefazolin for Injection and Dextrose Injection preoperatively, intraoperatively, and postoperatively may reduce the incidence of certain postoperative infections in patients undergoing surgical procedures which are classified as contaminated or potentially contaminated (e.g., vaginal hysterectomy, and cholecystectomy in high-risk patients such as those older than 70 years, with acute cholecystitis, obstructive jaundice, or common duct bile stones).
1.10 Usage
To reduce the development of drug-resistant bacteria and maintain the effectiveness of Cefazolin for Injection and Dextrose Injection and other antibacterial drugs, Cefazolin for Injection and Dextrose Injection should be used only to treat or prevent infections that are proven or strongly suspected to be caused by susceptible bacteria. When culture and susceptibility information are available, they should be considered in selecting or modifying antibacterial therapy. In the absence of such data, local epidemiology and susceptibility patterns may contribute to the empiric selection of therapy.
2. Cefazolin and Dextrose Dosage and Administration
2.1 Important Administration Instructions
If a dose of Cefazolin for Injection and Dextrose Injection is required that does not equal 1 gram, 2 grams, or 3 grams, this product is not recommended for use and an alternative formulation of cefazolin should be considered.
Administer Cefazolin for Injection and Dextrose Injection intravenously over approximately 30 minutes.
2.2 Dosage for the Treatment of Infections
Dosage for the Treatment of Infections in Adults with Creatinine Clearance (CLcr) Equal to 55 mL/min or Greater
The recommended adult dosages for the treatment of infections [see Indications and Usage (1.1 to 1.8)] are outlined in Table 1 below. Administer Cefazolin for Injection and Dextrose Injection intravenously over approximately 30 minutes.
|
||
| Table 1: Recommended Dosage in Adult Patients with CLcr Equal to 55 mL/min or Greater*. | ||
| Site and Type of Infection | Dose | Frequency |
| Moderate to severe infections | 500 mg to 1 gram | every 6 to 8 hours |
| Mild infections caused by susceptible gram-positive cocci | 250 mg to 500 mg | every 8 hours |
| Acute, uncomplicated urinary tract infections | 1 gram | every 12 hours |
| Pneumococcal pneumonia | 500 mg | every 12 hours |
| Severe, life-threatening infections (e.g., endocarditis, septicemia)† | 1 gram to 1.5 grams | every 6 hours |
Dosage for the Treatment of Infections in Pediatric Patients with CLcr Equal to 70 mL/min or Greater
The recommended pediatric dosages for the treatment of infections [see Indications and Usage (1.1 to 1.8)] are outlined in Table 2 below. Administer Cefazolin for Injection and Dextrose Injection intravenously over approximately 30 minutes.
If a dose of Cefazolin for Injection and Dextrose Injection is required that does not equal 1 gram, 2 grams or 3 grams, this product is not recommended for use and an alternative formulation of cefazolin should be considered [see Use in Specific Populations (8.4)].
| Table 2: Recommended Dosage in Pediatric Patients with CLcr 70 mL/min or greater for Treatment of Infections [see Indications and Usage (1.1 to 1.8)] | |
| Type of Severity | Recommended Total Daily Dosage |
| Mild to moderate infections | 25 mg/kg to 50 mg/kg, divided into 3 or 4 equal doses |
| Severe infections | May increase to 100 mg/kg, divided into 3 or 4 equal doses |
2.3 Dosage for Perioperative Prophylaxis
Dosage for Perioperative Prophylaxis in Adults with CLcr Equal to 55 mL/min or Greater
To prevent postoperative infection in contaminated or potentially contaminated surgery, recommended dosages are described in Table 3 below.
|
Table 3: Recommended Dosage for Perioperative Prophylaxis in Adults with CLcr Equal to 55 mL/min or Greater |
|||
| Body weight (kg)
| Dose administered
1/2 hour to 1 hour prior to the start of surgery | Additional dose during lengthy operative procedures (e.g., 2 hours or more) | Dose for 24 hours postoperatively |
| Less than 120 kg | 1 gram to 2 grams | 500 mg to 1 gram | 500 mg to 1 gram every 6 hours to 8 hours |
| Greater than or equal to 120 kg | 3 grams | ||
If a dose of Cefazolin for Injection and Dextrose Injection is required that does not equal 1 gram, 2 grams, or 3 grams, this product is not recommended and an alternative formulation of cefazolin should be considered.
It is important that (i) the preoperative dose be given just prior (1/2 hour to 1 hour) to the start of surgery so that adequate antibacterial concentrations are present in the serum and tissues at the time of initial surgical incision; and (ii) cefazolin be administered, if necessary, at appropriate intervals during surgery to provide sufficient concentrations of the antibacterial drug at the anticipated moments of greatest exposure to infective organisms.
The perioperative prophylactic administration of cefazolin should usually be discontinued within a 24-hour period after the surgical procedure. In surgery where the occurrence of infection may be particularly devastating (e.g., open-heart surgery and prosthetic arthroplasty), the prophylactic administration of cefazolin may be continued for 3 days to 5 days following the completion of surgery.
Dosage for Perioperative Prophylaxis in Pediatric Patients Aged 10 to 17 Years Old with CLcr 70 mL/min or Greater
To prevent postoperative infection in contaminated or potentially contaminated surgery, recommended doses are described in Table 4 below.
|
|||
| Table 4: Recommended Dosage for Perioperative Prophylaxis in Pediatric Patients with CLcr 70 mL/min or greater Aged 10 to 17 years Old* | |||
| Body weight (kg) | Dose administered 1/2 to 1 hour prior to the start of surgery | Additional dose during lengthy operative procedures (e.g., 2 hours or more) | Dose for 24 hours postoperatively |
| Less than 50 kg | 1 gram | 500 mg to 1 gram | 500 mg to 1 gram every 6 hours to 8 hours |
| Greater than or equal to 50 kg and less than 120 kg | 2 grams | ||
It is important that (i) the preoperative dose be given just prior (1/2 hour to 1 hour) to the start of surgery so that adequate antibacterial concentrations are present in the serum and tissues at the time of initial surgical incision; and (ii) cefazolin be administered, if necessary, at appropriate intervals during surgery to provide sufficient concentrations of the antibacterial drug at the anticipated moments of greatest exposure to infective organisms.
The administration of Cefazolin for Injection and Dextrose Injection for perioperative prophylaxis should usually be discontinued within a 24-hour period after the surgical procedure. In surgery where the occurrence of infection may be particularly devastating the administration of Cefazolin for Injection and Dextrose Injection for perioperative prophylaxis may be continued for 3 days to 5 days following the completion of surgery.
2.4 Dosage Recommendations in Adult and Pediatric Patients with Renal Impairment
Dosage Recommendations in Adult Patients with CLcr less than 55 mL/min
The dosage recommendation for Cefazolin for Injection and Dextrose Injection in adult patients with renal impairment (CLcr less than 55 mL/min) is outlined in Table 5 below.
|
||
| Table 5: Dosage Recommendation for Adult Patients with CLcr less than 55 mL/min | ||
| Creatinine Clearance | Dose | Frequency |
| 35 to 54 mL/min | Recommended dose | every 8 hours or longer |
| 11 to 34 mL/min | Half of recommended dose* | every 12 hours |
| 10 mL/min or less | Half of recommended dose* | every 18 to 24 hours |
Dosage Recommendations in Pediatric Patients with CLcr less than 70 mL/min
The dosage recommendation for Cefazolin for Injection and Dextrose Injection in pediatric patients with renal impairment (CLcr less than 70 mL/min) is outlined in Table 6 below.
| Table 6: Recommended Dosage in Pediatric Patients with CLcr less than 70 mL/min | |
| Creatinine Clearance | Recommended Dosage |
| 40 to 70 mL/min | 60% of the normal daily dose given in equally divided doses every 12 hours |
| 20 to 40 mL/min | 25% of the normal daily dose given in equally divided doses every 12 hours |
| 5 to 20 mL/min | 10% of the normal daily dose every 24 hours |
*If a dose of Cefazolin for Injection and Dextrose Injection is required that does not equal 1 gram, 2 grams, or 3 grams, this product is not recommended for use and an alternative formulation of cefazolin should be considered.
2.5 Preparation for Use of Cefazolin for Injection and Dextrose Injection in DUPLEX® Container
This reconstituted solution of Cefazolin for Injection and Dextrose Injection is for intravenous use only.
Do not use plastic containers in series connections. Such use would result in air embolism due to residual air being drawn from the primary container before administration of the fluid from the secondary container is complete. If administration is controlled by a pumping device, care must be taken to discontinue pumping action before the container runs dry or air embolism may result.
Parenteral drug products should be inspected visually for particulate matter and discoloration prior
to administration. Use only if solution is clear and container and seals are intact.
DUPLEX® Container Storage
- To avoid inadvertent activation, the DUPLEX® Container should remain in the folded position until activation is intended.
Patient Labeling and Drug Powder/Diluent Inspection
- Apply patient-specific label on foil side of container. Use care to avoid activation. Do not cover any portion of foil strip with patient label.
- Unlatch side tab and unfold DUPLEX® Container (see Diagram 1).
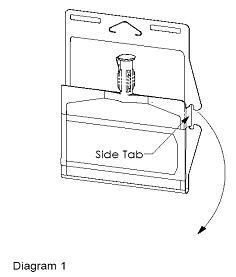
- Visually inspect diluent chamber for particulate matter.
- Use only if container and seals are intact.
- To inspect the drug powder for foreign matter or discoloration, peel foil strip from drug chamber (see Diagram 2).
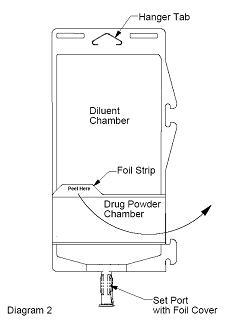
- Protect from light after removal of foil strip.
Note: If foil strip is removed, the container should be re-folded and the side tab latched until ready to activate. The product must then be used within 7 days, but not beyond the labeled expiration date.
Reconstitution (Activation)
- Do not use directly after storage by refrigeration, allow the product to equilibrate to room temperature before patient use.
- Unfold the DUPLEX® Container and point the set port in a downward direction. Starting at the hanger tab end, fold the DUPLEX® Container just below the diluent meniscus trapping all air above the fold. To activate, squeeze the folded diluent chamber until the seal between the diluent and powder opens, releasing diluent into the drug powder chamber (see Diagram 3).
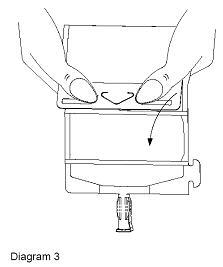
- Agitate the liquid-powder mixture until the drug powder is completely dissolved.
Note: Following reconstitution (activation), product must be used within 24 hours if stored at room temperature or within 7 days if stored under refrigeration.
Administration
- Visually inspect the reconstituted solution for particulate matter.
- Point the set port in a downwards direction. Starting at the hanger tab end, fold the DUPLEX® Container just below the solution meniscus trapping all air above the fold. Squeeze the folded DUPLEX® Container until the seal between reconstituted drug solution and set port opens, releasing liquid to set port (see Diagram 4).
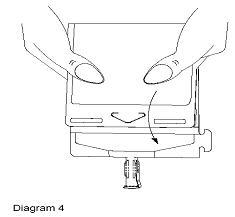
- Prior to attaching the IV set, check for minute leaks by squeezing container firmly. If leaks are found, discard container and solution as sterility may be compromised.
- Using aseptic technique, peel foil cover from the set port and attach sterile administration set (see Diagram 5).
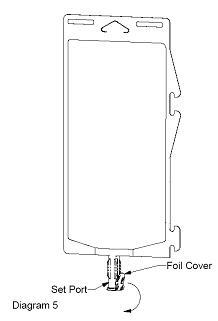
- Refer to directions for use accompanying the administration set.
Important Administration Instructions
- Do not use in series connections.
- Do not introduce additives into the DUPLEX® Container.
- Administer Cefazolin for Injection and Dextrose Injection intravenously over approximately 30 minutes.
3. Dosage Forms and Strengths
Dual-chamber, single-dose packaged combination of Cefazolin Sodium USP (lyophilized) and sterile iso-osmotic diluent in the DUPLEX® sterile container consisting of:
- 1 gram Cefazolin for Injection USP and 50 mL 4% Dextrose Injection USP
- 2 grams Cefazolin for Injection USP and 50 mL 3% Dextrose Injection USP
- 3 grams Cefazolin for Injection USP and 50 mL 2% Dextrose Injection USP
4. Contraindications
4.1 Hypersensitivity to Cefazolin or the Cephalosporin Class of Antibacterial Drugs, Penicillins, or Other Beta-lactams
Cefazolin for Injection and Dextrose Injection is contraindicated in patients who have a history of immediate hypersensitivity reactions (e.g., anaphylaxis, serious skin reactions) to cefazolin or the cephalosporin class of antibacterial drugs, penicillins, or other beta-lactams [see Warnings and Precautions (5.1)].
5. Warnings and Precautions
5.1 Hypersensitivity Reactions to Cefazolin, Cephalosporins, Penicillins, or Other Beta-lactams
Serious and occasionally fatal hypersensitivity (anaphylactic) reactions have been reported in patients receiving beta-lactam antibacterial drugs. Before therapy with Cefazolin for Injection and Dextrose Injection is instituted, careful inquiry should be made to determine whether the patient has had previous immediate hypersensitivity reactions to cefazolin, cephalosporins, penicillins, or carbapenems. Exercise caution if this product is to be given to penicillin-sensitive patients because cross-hypersensitivity among beta-lactam antibacterial drugs has been clearly documented and may occur in up to 10% of patients with a history of penicillin allergy. If an allergic reaction to Cefazolin for Injection and Dextrose Injection occurs, discontinue the drug.
5.2 Seizures in Patients with Renal Impairment
Seizures may occur with the administration of Cefazolin for Injection and Dextrose Injection, particularly in patients with renal impairment when the dosage is not reduced appropriately. Discontinue Cefazolin for Injection and Dextrose Injection if seizures occur or make appropriate dosage adjustments in patients with renal impairment [see Dosage and Administration (2.4)]. Anticonvulsant therapy should be continued in patients with known seizure disorders.
5.3 Clostridioides difficile-associated Diarrhea
Clostridioides difficile-associated diarrhea (CDAD) has been reported with use of nearly all antibacterial agents, including cefazolin, and may range in severity from mild diarrhea to fatal colitis. Treatment with antibacterial agents alters the normal flora of the colon leading to overgrowth of C. difficile.
C. difficile produces toxins A and B, which contribute to the development of CDAD. Hypertoxin-producing isolates of C. difficile cause increased morbidity and mortality, as these infections can be refractory to antimicrobial therapy and may require colectomy. CDAD must be considered in all patients who present with diarrhea following antibacterial drug use. Careful medical history is necessary since CDAD has been reported to occur over two months after the administration of antibacterial agents.
If CDAD is suspected or confirmed, ongoing antibacterial drug use not directed against C. difficile may need to be discontinued. Appropriate fluid and electrolyte management, protein supplementation, antibacterial drug treatment of C. difficile, and surgical evaluation should be instituted as clinically indicated.
5.4 Hypersensitivity to Dextrose-containing Products
Hypersensitivity reactions, including anaphylaxis, have been reported with administration of dextrose-containing products. These reactions have been reported in patients receiving high concentrations of dextrose (i.e. 50% dextrose)1. The reactions have also been reported when corn-derived dextrose solutions were administered to patients with or without a history of hypersensitivity to corn products.2
5.5 Prothrombin Activity
Cefazolin for Injection and Dextrose Injection may be associated with a fall in prothrombin activity. Those at risk include patients with renal or hepatic impairment or poor nutritional state, as well as patients receiving a protracted course of antimicrobial therapy, and patients previously stabilized on anticoagulant therapy. Prothrombin time should be monitored in patients at risk and exogenous vitamin K administered as indicated.
5.6 Risk of Development of Drug-resistant Bacteria
Prescribing Cefazolin for Injection and Dextrose Injection in the absence of proven or strongly suspected bacterial infection or a prophylactic indication is unlikely to provide benefit to the patient and increases the risk of the development of drug-resistant bacteria.
As with other antimicrobials, prolonged use of Cefazolin for Injection and Dextrose Injection may result in overgrowth of nonsusceptible microorganisms. Repeated evaluation of the patient's condition is essential. Should superinfection occur during therapy, appropriate measures should be taken.
5.7 Drug/Laboratory Test Interactions
Urinary Glucose
The administration of cefazolin may result in a false-positive reaction with glucose in the urine when using glucose tests based on Benedict’s copper reduction reaction that determine the amount of reducing substances like glucose in the urine. It is recommended that glucose tests based on enzymatic glucose oxidase be used.
Coombs’ Test
Positive direct Coombs' tests have been reported during treatment with cefazolin. In hematologic studies or in transfusion cross-matching procedures when antiglobulin tests are performed on the minor side or in Coombs' testing of newborns whose mothers have received cephalosporin antibacterial drugs before parturition, it should be recognized that a positive Coombs' test may be due to the drug.
5.8 Patients with Overt or Known Subclinical Diabetes Mellitus or Carbohydrate Intolerance
As with other dextrose-containing solutions, Cefazolin for Injection and Dextrose Injection should be prescribed with caution in patients with overt or known subclinical diabetes mellitus or carbohydrate intolerance for any reason.
6. Adverse Reactions/Side Effects
The following serious adverse reactions to Cefazolin for Injection and Dextrose Injection are described below and elsewhere in the labeling:
- Hypersensitivity Reactions to Cefazolin, Cephalosporins, Penicillins, or Other Beta-lactams [see Warnings and Precautions (5.1)]
- Seizures in Patients with Renal Impairment [see Warnings and Precautions (5.2)]
- Clostridioides difficile-associated Diarrhea [see Warnings and Precautions (5.3)]
- Prothrombin Activity [see Warnings and Precautions (5.5)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The following adverse reactions were reported from clinical trials:
Gastrointestinal: Diarrhea, oral candidiasis (oral thrush), mouth ulcers, vomiting, nausea, stomach cramps, epigastric pain, heartburn, flatus, anorexia and pseudomembranous colitis. Onset of pseudomembranous colitis symptoms may occur during or after antibacterial treatment [see Warnings and Precautions (5.3)].
Allergic: Anaphylaxis, eosinophilia, urticaria, itching, drug fever, skin rash, Stevens-Johnson syndrome.
Hematologic: Neutropenia, leukopenia, thrombocytopenia, thrombocythemia.
Hepatic: Transient rise in SGOT, SGPT, and alkaline phosphatase levels has been observed. Reports of hepatitis have been received.
Renal: Reports of increased BUN and creatinine levels, as well as renal failure, have been received.
Local Reactions: Instances of phlebitis have been reported at site of injection. Some induration has occurred.
Other Reactions: Pruritus (including genital, vulvar and anal pruritus, genital moniliasis, and vaginitis). Dizziness, fainting, lightheadedness, confusion, weakness, tiredness, hypotension, somnolence and headache.
Adverse Reactions in Pediatric Patients for Perioperative Prophylaxis
Two studies (Study 1: NCT 3231228 and Study 2: NCT 01904357) were conducted to assess the safety and pharmacokinetics of a single 30-minute infusion of either 1 gram or 2 grams (based on weight) of Cefazolin for Injection and Dextrose Injection for perioperative prophylaxis in pediatric patients.
Study 1 was a multicenter, open-label, non-comparative, parallel group study to evaluate the safety and pharmacokinetics of a single 30-minute infusion of either 1 gram or 2 grams (based on weight) of Cefazolin for Injection and Dextrose Injection for perioperative prophylaxis in 61 pediatric patients 10 to 17 years of age. Thirty-three subjects with a weight of at least 25 kg but less than 60 kg received a single-dose of 1 gram of cefazolin and 28 subjects with a weight of at least 60 kg received a single-dose of 2 grams of cefazolin. The mean age of the safety population was 14 years and ranged from 10 to 17 years. There were no adverse reactions leading to study discontinuation or deaths reported during the study. The most frequently reported adverse reactions were nausea (14.8%), infusion site pain (6.6%), and headache (4.9%).
Study 2 was a multicenter, non-comparative study that evaluated the safety and pharmacokinetics of a single 30-minute infusion of either 1 gram or 2 grams (based on weight) of Cefazolin for Injection and Dextrose Injection for perioperative prophylaxis in 12 pediatric patients 10 to 12 years of age. Subjects weighing at least 25 kg to less than 50 kg received a single-dose of 1 gram of Cefazolin for Injection and Dextrose Injection and subjects weighing at least 50 kg to less than 85 kg received a single-dose of 2 grams of Cefazolin for Injection and Dextrose Injection.
The safety findings in Study 2 in pediatric patients aged 10 to 12 years old were similar to those observed in adult patients and the pediatric patients aged 10 to 17 years old in Study 1.
6.2 Postmarketing Experience
The following adverse reactions have been identified during post approval use of cefazolin. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Immune system disorders: Serum sickness-like reaction
Renal and urinary disorders: Acute tubulointerstitial nephritis (ATIN)
Skin and subcutaneous tissue disorders: Acute generalized exanthematous pustulosis (AGEP)
6.3 Cephalosporin-class Adverse Reactions
In addition to the adverse reactions listed above that have been observed in patients treated with cefazolin, the following adverse reactions and altered laboratory tests have been reported for cephalosporin-class antibacterials:
Stevens-Johnson syndrome, erythema multiforme, toxic epidermal necrolysis, renal impairment, toxic nephropathy, aplastic anemia, hemolytic anemia, hemorrhage, a fall in prothrombin activity, hepatic impairment including cholestasis, and pancytopenia.
7. Drug Interactions
The renal excretion of cefazolin is inhibited by probenecid. Co-administration of probenecid with Cefazolin for Injection and Dextrose Injection is not recommended.
8. Use In Specific Populations
8.1 Pregnancy
Risk Summary
Available data from published prospective cohort studies, case series and case reports over several decades with cephalosporin use, including cefazolin, in pregnant women have not established a drug-associated risk of major birth defects, miscarriage, or adverse maternal or fetal outcomes. Cefazolin crosses the placenta.
Animal reproduction studies with rats, mice and rabbits administered cefazolin during organogenesis at doses 1 to 3 times the maximum recommended human dose (MRHD) did not demonstrate adverse developmental outcomes. In rats subcutaneously administered cefazolin prior to delivery and throughout lactation, there were no adverse effects on offspring at a dose approximately 2 times the MRHD (see Data).
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
Data
Human Data
While available studies cannot definitively establish the absence of risk, published data from case-control studies and case reports over several decades have not identified an association with cephalosporin use during pregnancy and major birth defects, miscarriage, or other adverse maternal or fetal outcomes. Available studies have methodologic limitations, including small sample size, retrospective data collection, and inconsistent comparator groups.
Animal Data
Reproduction studies have been performed in rats, mice and rabbits administered cefazolin during organogenesis at doses of 2000, 4000 and 240 mg/kg/day (approximately 1 to 3 times the maximum recommended human dose on a body surface area comparison). There was no evidence of any adverse effects on embryofetal development due to cefazolin. In a peri-postnatal study in rats, cefazolin administered subcutaneously up to 1200 mg/kg/day (approximately 2 times the MRHD based on body surface area comparison) to pregnant dams prior to delivery and through lactation caused no adverse effects on offspring.
8.2 Lactation
Data from published literature report that cefazolin is present in human milk, but is not expected to accumulate in a breastfed infant. There are no data on the effects of cefazolin on the breastfed child or on milk production.
The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for Cefazolin for Injection and Dextrose Injection and any potential adverse effects on the breastfed child from Cefazolin for Injection and Dextrose Injection or from the mother’s underlying condition.
8.4 Pediatric Use
Cefazolin for Injection and Dextrose Injection is indicated for the treatment of respiratory tract infections, urinary tract infections, skin and skin structure infections, biliary tract infections, bone and joint infections, genital infections, septicemia, and endocarditis in pediatric patients for whom appropriate dosing with this formulation can be achieved, and for perioperative prophylaxis in pediatric patients aged 10 to 17 years old [see Indications and Usage (1.1 to 1.9)].
Safety and effectiveness of Cefazolin for Injection and Dextrose Injection in premature infants and neonates have not been established and is not recommended for use in this age group of pediatric patients. Dosing for cefazolin in pediatric patients younger than one month old has not been established.
Because of the limitations of the available strengths and administration requirements (i.e., administration of fractional doses is not recommended) of Cefazolin for Injection and Dextrose Injection, and to avoid unintentional overdose, this product is not recommended for use if a dose of Cefazolin for Injection and Dextrose Injection that does not equal 1 gram or 2 grams is required and an alternative formulation of cefazolin should be considered [see Dosage and Administration (2.2, 2.3, 2.4 and 2.5)].
The safety and effectiveness of Cefazolin for Injection and Dextrose Injection for perioperative prophylaxis have been established in pediatric patients aged 10 to 17 years old. Use of Cefazolin for Injection and Dextrose Injection in these age groups is supported by evidence from adults with additional safety and pharmacokinetic data in pediatric patients aged 10 to 17 years old. Safety and pharmacokinetics were evaluated in two multicenter, non-comparative studies (Study 1 and Study 2). These studies were conducted to assess the safety and pharmacokinetics of a single 30-minute infusion of either 1 gram or 2 grams (based on weight) of Cefazolin for Injection and Dextrose Injection for perioperative prophylaxis in pediatric patients. Study 1 evaluated the safety and pharmacokinetics of 1 gram of Cefazolin for Injection and Dextrose Injection in pediatric patients aged 10 to 17 years old scheduled for surgery with a weight of at least 25 kg but less than 60 kg and, 2 grams in pediatric patients with a weight of at least 60 kg. Study 2 evaluated 1 gram of Cefazolin for Injection and Dextrose Injection in pediatric patients aged 10 to 12 years old scheduled for surgery with a weight of at least 25 kg but less than 50 kg and, 2 grams in pediatric patients with a weight of at least 50 kg to less than 85 kg [see Dosage and Administration (2.3), Adverse Reactions (6.1) and Clinical Pharmacology (12.3)].
The safety and effectiveness of Cefazolin for Injection and Dextrose Injection for perioperative prophylaxis have not been established in pediatric patients younger than 10 years old.
8.5 Geriatric Use
Of the 920 subjects who received cefazolin in clinical studies, 313 (34%) were 65 years and over, while 138 (15%) were 75 years and over. No overall differences in safety or effectiveness were observed between these subjects and younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients, but greater sensitivity of some older individuals cannot be ruled out.
This drug is known to be substantially excreted by the kidney, and the risk of toxic reactions to this drug may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection, and it may be useful to monitor renal function [see Dosage and Administration (2.4) and Warnings and Precautions (5.2)].
8.6 Patients with Renal Impairment
When Cefazolin for Injection and Dextrose Injection is administered to adult and pediatric patients with low urinary output because of impaired renal function (creatinine clearance less than 55 mL/min and 70 mL/min for adults and pediatric patients, respectively), lower daily dosage is required [see Dosage and Administration (2.4) and Warnings and Precautions (5.2)].
10. Overdosage
Accidental overdosage resulting in seizures may occur in patients with renal impairment who receive doses greater than the recommended dosage of Cefazolin for Injection and Dextrose Injection [see Warnings and Precautions (5.2)]. If seizures associated with accidental overdosage occur, discontinue Cefazolin for Injection and Dextrose Injection and give supportive treatment.
11. Cefazolin and Dextrose Description
Cefazolin for Injection USP and Dextrose Injection USP is a sterile, nonpyrogenic, single-dose, packaged combination of Cefazolin Sodium USP (lyophilized) and sterile iso-osmotic diluent in the DUPLEX® sterile container. The DUPLEX® Container is a flexible dual chamber container.
After reconstitution the approximate osmolality for Cefazolin for Injection USP and Dextrose Injection USP is 290 mOsmol/kg.
The drug chamber is filled with sterile lyophilized Cefazolin Sodium USP, a semi-synthetic cephalosporin and has the following IUPAC nomenclature: Sodium (6R,7R)-3-[[(5-methyl-1,3,4-thiadiazol-2-yl)thio]methyl]-8-oxo-7-[2-(1H-tetrazol-1-yl)acetamido]-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylate. Its empirical formula is C14H13N8NaO4S3 and its molecular weight is 476.48.
Cefazolin Sodium USP has the following structural formula:
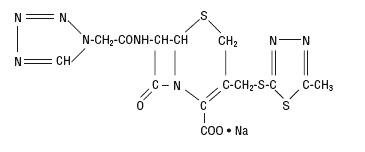
The sodium content is 48 mg/g of cefazolin sodium.
The diluent chamber contains Dextrose Injection USP, an iso-osmotic diluent using Hydrous Dextrose USP in Water for Injection USP. Dextrose Injection USP is sterile, nonpyrogenic, and contains no bacteriostatic or antimicrobial agents. Its empirical formula is C6H12O6•H2O and its molecular weight is 198.17.
Hydrous Dextrose USP has the following structural (molecular) formula:
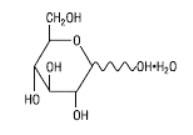
Cefazolin Sodium USP is supplied as a lyophilized form equivalent to either 1 gram, 2 grams or 3 grams of cefazolin. The 1 gram / container Cefazolin Sodium USP contains 1 gram of cefazolin (equivalent to 1.048 grams of cefazolin sodium). The 2 grams / container Cefazolin Sodium USP contains 2 grams of cefazolin (equivalent to 2.096 grams of cefazolin sodium). The 3 grams / container Cefazolin Sodium USP contains 3 grams of cefazolin (equivalent to 3.145 grams of cefazolin sodium). Dextrose Hydrous USP has been added to the diluent (Water for Injection USP) to adjust osmolality (approximately 2 grams [4% w/v], 1.5 grams [3% w/v] and 1 gram [2% w/v] for the 1 gram, 2 gram or 3 gram dosages, respectively).
After removing the peelable foil strip, activating the seals, and thoroughly mixing, the reconstituted drug product is intended for single intravenous use.
The pH of reconstituted solutions of Cefazolin for Injection and Dextrose Injection is 3.5 to 7.0.
Reconstituted solutions of Cefazolin for Injection and Dextrose Injection range in color from pale yellow to amber.
Not made with natural rubber latex, PVC or DEHP.
The DUPLEX® dual chamber container is made from a specially formulated material. The product (diluent and drug) contact layer is a mixture of thermoplastic rubber and a polypropylene ethylene copolymer that contains no plasticizers. The safety of the container system is supported by USP biological evaluation procedures.
12. Cefazolin and Dextrose - Clinical Pharmacology
12.2 Pharmacodynamics
The pharmacokinetic/pharmacodynamic relationship for cefazolin has not been evaluated in patients.
12.3 Pharmacokinetics
Studies have shown that following intravenous administration of cefazolin to normal volunteers, mean serum concentrations peaked at approximately 185 mcg/mL and were approximately 4 mcg/mL at 8 hours for a 1 gram dose.
The serum half-life for cefazolin is approximately 1.8 hours following IV administration.
In a study of constant intravenous infusion with dosages of 3.5 mg/kg for 1 hour (approximately 250 mg) and 1.5 mg/kg the next 2 hours (approximately 100 mg) in healthy volunteers, cefazolin serum concentrations at the third hour were approximately 28 mcg/mL.
Plasma pharmacokinetic parameters of cefazolin in healthy volunteers (N=12) following a single 15-minute IV infusion of 2 grams of Cefazolin for Injection and Dextrose Injection are summarized in Table 7.
|
|||||||
| Table 7: Mean (Standard Deviation) Plasma Pharmacokinetic Parameters of Cefazolin in Healthy Volunteers | |||||||
| Dosage of Cefazolin for Injection and Dextrose Injection | N |
Cmax (mcg/mL) |
Tmax* | AUC0-inf
(mcg*h/mL) |
t1/2
|
CL |
Vz
|
| Single 2 grams Dose as a 15-Minute IV Infusion | 12 | 280.9 (45.9) | 0.25 (0.25-0.33) | 509.9 (89.3) | 2.01 (0.28) | 4.03 (0.68) | 11.50 (1.53) |
N= number of subjects observed; Cmax = maximum plasma concentration; Tmax = time to maximum plasma concentration; AUC0-inf = area under the plasma concentration-time curve extrapolated to infinity; t1/2 = apparent plasma terminal elimination half-life; CL = total clearance; Vz = volume of distribution
Model derived plasma pharmacokinetic parameters of cefazolin in adult patients weighing 120 kg or greater (N=12) following a single 30-minute IV infusion of 3 grams of Cefazolin for Injection and Dextrose Injection are summarized in Table 8.
| Table 8: Mean (Standard Deviation) Model Derived Plasma Pharmacokinetic Parameters of Cefazolin in Adult Patients Weighing ≥ 120 kg |
|||||||
| Dosage of Cefazolin for Injection and Dextrose Injection | N | Cmax
(mcg/mL) | Tmax* (h) | AUC0--inf
(mcg*h/mL) | t1/2
(h) | CL (L/h) | Vz (L) |
| Single 3 grams Dose as a 30-Minute IV Infusion | 12 | 197.7 (46.6) | 0.5 | 598.6 (206.6) | 2.36 (0.687) | 5.52 (1.72) | 17.51 (3.63) |
* Tmax reported based on infusion duration
N= number of subjects observed; Cmax = maximum plasma concentration; Tmax = time to maximum plasma concentration; AUC0-inf = area under the plasma concentration-time curve extrapolated to infinity; t1/2 = apparent plasma terminal elimination half-life; CL = total clearance; Vz = volume of distribution
Studies in patients hospitalized with infections indicate that cefazolin mean peak serum concentrations were approximately equivalent to those seen in healthy volunteers.
Bile concentrations in patients without obstructive biliary disease can reach or exceed serum concentrations by up to five times; however, in patients with obstructive biliary disease, bile concentrations of cefazolin are considerably lower than serum concentrations (less than 1.0 mcg/mL).
In synovial fluid, the cefazolin concentration becomes comparable to that reached in serum at about 4 hours after drug administration.
Studies of cord blood show prompt transfer of cefazolin across the placenta. Cefazolin is present in very low concentrations in the milk of nursing mothers.
Cefazolin is excreted unchanged in the urine. In the first 6 hours, approximately 60% of the drug is excreted in the urine and this increases to 70% to 80% within 24 hours.
Specific Populations
Pediatric Patients for Perioperative Prophylaxis
A simulation based on pharmacokinetic data from healthy adults (n=24), pediatric patients aged 10 to 17 years (n=26: Study 1 [see Adverse Reactions (6.1)]), and pediatric patients aged 10 to 12 years (n=12: Study 2 [see Adverse Reactions (6.1)] indicate that the administration of a 1 gram cefazolin dose for pediatric patients weighing less than 50 kg and a 2 gram cefazolin dose for those weighing 50 kg or greater will provide comparable exposures between pediatric patients aged 10 to 17 years and healthy adults receiving 2 grams Cefazolin for Injection and Dextrose Injection [see Dosage and Administration (2.2 and 2.3) and Use in Specific Populations (8.4)].
12.4 Microbiology
Mechanism of Action
Cefazolin is a bactericidal agent that acts by inhibition of bacterial cell wall synthesis.
Resistance
Predominant mechanisms of bacterial resistance to cephalosporins include the presence of extended-spectrum beta-lactamases and enzymatic hydrolysis.
Antimicrobial Activity
Cefazolin has been shown to be active against most isolates of the following microorganisms, both in vitro and in clinical infections [see Indications and Usage (1)].
Gram-positive bacteria
- Staphylococcus aureus
- Staphylococcus epidermidis
- Streptococcus agalactiae
- Streptococcus pneumoniae
- Streptococcus pyogenes
Methicillin-resistant staphylococci are uniformly resistant to cefazolin.
Gram-negative bacteria
- Escherichia coli
- Proteus mirabilis
Most isolates of indole positive Proteus (Proteus vulgaris), Enterobacter spp., Morganella morganii, Providencia rettgeri, Serratia spp., and Pseudomonas spp. are resistant to cefazolin.
13. Nonclinical Toxicology
15. References
- Czarny D, Prichard PJ, Fennessy M, Lewis S. Anaphylactoid reaction to 50% solution of dextrose. Med J Aust 1980;2:255-258.
- Guharoy, SR, Barajas M. Probably Anaphylactic Reaction to Corn-Derived Dextrose Solution. Vet Hum Toxicol 1991;33:609-610.
16. How is Cefazolin and Dextrose supplied
Cefazolin for Injection USP and Dextrose Injection USP in the single-dose DUPLEX® Container is a flexible dual chamber container supplied in three concentrations. After reconstitution, the concentrations are equivalent to either 1 gram, 2 grams or 3 grams of cefazolin. The diluent chamber contains approximately 50 mL of Dextrose Injection USP. Dextrose Injection USP has been adjusted to 4%, 3% and 2% for the 1 gram, 2 gram and 3 gram doses, respectively, such that the reconstituted solution is iso-osmotic.
Cefazolin for Injection USP and Dextrose Injection USP is supplied sterile and nonpyrogenic in the DUPLEX® Container packaged 24 units per case.
| NDC | REF | Dose | Volume |
| 0264-3103-11 | 3103-11 | 1 gram | 50 mL |
| 0264-3105-11 | 3105-11 | 2 grams | 50 mL |
| 0264-3107-11 | 3107-11 | 3 grams | 50 mL |
Store the unactivated unit at 20-25°C (68-77°F). Excursions permitted to 15-30°C (59-86°F). [See USP Controlled Room Temperature.] Do not freeze.
As with other cephalosporins, reconstituted Cefazolin for Injection USP and Dextrose Injection USP tends to darken depending on storage conditions, within the stated recommendations. However, product potency is not adversely affected.
Use only if prepared solution is clear and free from particulate matter [see Dosage and Administration (2.5)].
17. Patient Counseling Information
Serious Allergic Reactions
Advise patients that allergic reactions, including serious allergic reactions could occur and that serious reactions require immediate treatment and discontinuation of Cefazolin for Injection and Dextrose Injection. Patients should report to their health care provider any previous allergic reactions to cefazolin, cephalosporins, penicillins, or other similar antibacterials [see Warnings and Precautions (5.1)].
Seizures
Advise patients that seizures could occur with Cefazolin for Injection and Dextrose Injection. Instruct patients to inform a healthcare provider at once of any signs and symptoms of seizures, for immediate treatment, dosage adjustment, or discontinuation of Cefazolin for Injection and Dextrose Injection [see Warnings and Precautions (5.2)].
Diarrhea
Advise patients that diarrhea is a common problem caused by antibacterials, including Cefazolin for Injection and Dextrose Injection, which usually ends when the antibacterial is discontinued. Sometimes after starting treatment with antibacterials, patients can develop watery and bloody stools (with or without stomach cramps and fever) even as late as two or more months after having taken the last dose of the antibacterials. If this occurs, patients should contact a physician as soon as possible [see Warnings and Precautions (5.3)].
Antibacterial Resistance
Patients should be counseled that antibacterial drugs, including Cefazolin for Injection and Dextrose Injection should only be used to treat bacterial infections. They do not treat viral infections (e.g., the common cold). When Cefazolin for Injection and Dextrose Injection is prescribed to treat a bacterial infection, patients should be told that although it is common to feel better early in the course of therapy, the medication should be taken exactly as directed. Skipping doses or not completing the full course of therapy may (1) decrease the effectiveness of the immediate treatment and (2) increase the likelihood that bacteria will develop resistance and will not be treatable by Cefazolin for Injection and Dextrose Injection or other antibacterial drugs in the future.
DUPLEX is a registered trademark of B. Braun Medical Inc.
ATCC is a registered trademark of the American Type Culture Collection.
B. Braun Medical Inc.
Bethlehem, PA 18018-3524 USA
1-800-227-2862
Prepared in USA.
API from USA and Italy.
Y36-003-090 LD-105-12
PRINCIPAL DISPLAY PANEL - 1g Cefazolin
Cefazolin for Injection USP
and Dextrose Injection USP
1g*
50 mL
REF 3103-11
NDC 0264-3103-11
DUPLEX®CONTAINER
Use only after mixing contents of both chambers.
For IV Use Only Iso-osmotic Single Dose Sterile/Nonpyrogenic
* Contains Cefazolin Sodium USP equivalent to 1 g cefazolin.
Reconstitution: Hold container with set port in a downward direction and fold
the diluent chamber just below the solution meniscus. To activate seal, squeeze
folded diluent chamber until seal between diluent and drug chamber opens,
releasing diluent into drug chamber. Agitate the reconstituted solution until the
drug powder is completely dissolved. Fold the container a second time and
squeeze until seal between drug chamber and set port opens.
After reconstitution each 50 mL single dose unit contains: Cefazolin for Injection
USP (equivalent to 1 g cefazolin) with approx. 2.0 g (4.0% w/v) Hydrous Dextrose
USP in Water for Injection USP. Sodium content is 48 mg/g of cefazolin sodium.
Approximate osmolality: 290 mOsmol/kg
Prior to Reconstitution: Store at 20-25°C (68-77°F). Excursions permitted to
15-30°C (59-86°F). [See USP Controlled Room Temperature.] Use only if container
and seals are intact. Do not peel foil strip until ready for use. After foil strip
removal, product must be used within 7 days, but not beyond the labeled
expiration date. Protect from light after removal of foil strip.
After Reconstitution: Use only if prepared solution is clear and free from
particulate matter. Use within 24 hours if stored at room temperature or within
7 days if stored under refrigeration. Do not use in a series connection. Do not
introduce additives into this container. Prior to administration check for minute
leaks by squeezing container firmly. If leaks are found, discard container and
solution as sterility may be impaired. Do not freeze.
Not made with natural rubber latex, PVC or DEHP.
Rx only
B. Braun Medical Inc.
Bethlehem, PA 18018-3524
Prepared in USA. API from USA and Italy.
LD-201-7
Y37-002-541
EXP
LOT
PEEL HERE
Drug Chamber
Discard unit if foil strip is damaged. Peel foil strip only when
ready for use. Visually inspect drug prior to reconstitution.
See package insert for complete directions for reconstitution
and administration.
LD-336-1 X27-001-485


PRINCIPAL DISPLAY PANEL - 2g Cefazolin
Cefazolin for Injection USP
and Dextrose Injection USP
2g*
50 mL
REF 3105-11
NDC 0264-3105-11
DUPLEX®CONTAINER
Use only after mixing contents of both chambers.
For IV Use Only Iso-osmotic Single Dose Sterile/Nonpyrogenic
* Contains Cefazolin Sodium USP equivalent to 2 g cefazolin.
Reconstitution: Hold container with set port in a downward direction and fold
the diluent chamber just below the solution meniscus. To activate seal, squeeze
folded diluent chamber until seal between diluent and drug chamber opens,
releasing diluent into drug chamber. Agitate the reconstituted solution until the
drug powder is completely dissolved. Fold the container a second time
and squeeze until seal between drug chamber and set port opens.
After reconstitution each 50 mL single dose unit contains: Cefazolin for Injection
USP (equivalent to 2 g cefazolin) with approx. 1.5 g (3.0% w/v) Hydrous Dextrose
USP in Water for Injection USP. Sodium content is 48 mg/g of cefazolin sodium.
Approximate osmolality: 290 mOsmol/kg
Prior to Reconstitution: Store at 20-25°C (68-77°F). Excursions permitted to
15-30°C (59-86°F). [See USP Controlled Room Temperature.] Use only if container
and seals are intact. Do not peel foil strip until ready for use. After foil strip
removal, product must be used within 7 days, but not beyond the labeled
expiration date. Protect from light after removal of foil strip.
After Reconstitution: Use only if prepared solution is clear and free from
particulate matter. Use within 24 hours if stored at room temperature or within
7 days if stored under refrigeration. Do not use in a series connection. Do not
introduce additives into this container. Prior to administration check for minute
leaks by squeezing container firmly. If leaks are found, discard container and
solution as sterility may be impaired. Do not freeze.
Not made with natural rubber latex, PVC or DEHP.
Rx only
B. Braun Medical Inc.
Bethlehem, PA 18018-3524
Prepared in USA. API from USA and Italy.
LD-200-4
Y37-002-542
EXP
LOT
PEEL HERE
Drug Chamber
Discard unit if foil strip is damaged. Peel foil strip only when
ready for use. Visually inspect drug prior to reconstitution.
See package insert for complete directions for reconstitution
and administration.
LD-336-1 X27-001-485


PRINCIPAL DISPLAY PANEL - 3g Cefazolin
Cefazolin for Injection USP
and Dextrose Injection USP
3g*
50 mL
REF 3107-11
NDC 0264-3107-11
DUPLEX®CONTAINER
Use only after mixing contents of both chambers.
For IV Use Only Iso-osmotic Single-Dose Sterile/Nonpyrogenic
* Contains 3.145 g of Cefazolin Sodium USP equivalent to 3 g of cefazolin.
Reconstitution: Hold container with set port in a downward direction and fold
the diluent chamber just below the solution meniscus. To activate seal, squeeze
folded diluent chamber until seal between diluent and drug chamber opens,
releasing diluent into drug chamber. Agitate the reconstituted solution until the
drug powder is completely dissolved. Fold the container a second time and
squeeze until seal between drug chamber and set port opens.
After reconstitution each 50 mL single-dose unit contains: Cefazolin for Injection
USP (3 g cefazolin, equivalent to 3.145 g of cefazolin sodium) with approx. 1 g
(2% w/v) Hydrous Dextrose USP in Water for Injection USP.
Approximate osmolality: 290 mOsmol/kg
Prior to Reconstitution: Store at 20-25°C (68-77°F). Excursions permitted to
15-30°C (59-86°F). [See USP Controlled Room Temperature.] Use only if container
and seals are intact. Do not peel foil strip until ready for use. After foil strip
removal, product must be used within 7 days, but not beyond the labeled
expiration date. Protect from light after removal of foil strip.
After Reconstitution: Use only if prepared solution is clear and free from
particulate matter. Use within 24 hours if stored at room temperature or within
7 days if stored under refrigeration. Do not use in a series connection. Do not
introduce additives into this container. Prior to administration check for minute
leaks by squeezing container firmly. If leaks are found, discard container and
solution as sterility may be impaired. Do not freeze. Discard unused portion.
Not made with natural rubber latex, PVC or DEHP.
Rx only
B. Braun Medical Inc.
Bethlehem, PA 18018-3524
Prepared in USA. API from USA and Italy.
LD-792-2
Y37-002-609
EXP
LOT
PEEL HERE
Drug Chamber
Discard unit if foil strip is damaged. Peel foil strip only when
ready for use. Visually inspect drug prior to reconstitution.
See package insert for complete directions for reconstitution
and administration.
LD-336-1 X27-001-485


| CEFAZOLIN SODIUM
cefazolin sodium solution |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| CEFAZOLIN SODIUM
cefazolin sodium solution |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| CEFAZOLIN SODIUM
cefazolin sodium solution |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - B. Braun Medical Inc. (002397347) |
More about cefazolin
- Check interactions
- Compare alternatives
- Pricing & coupons
- Reviews (5)
- Latest FDA alerts (2)
- Side effects
- Dosage information
- During pregnancy
- Drug class: first generation cephalosporins
- Breastfeeding
- En español
