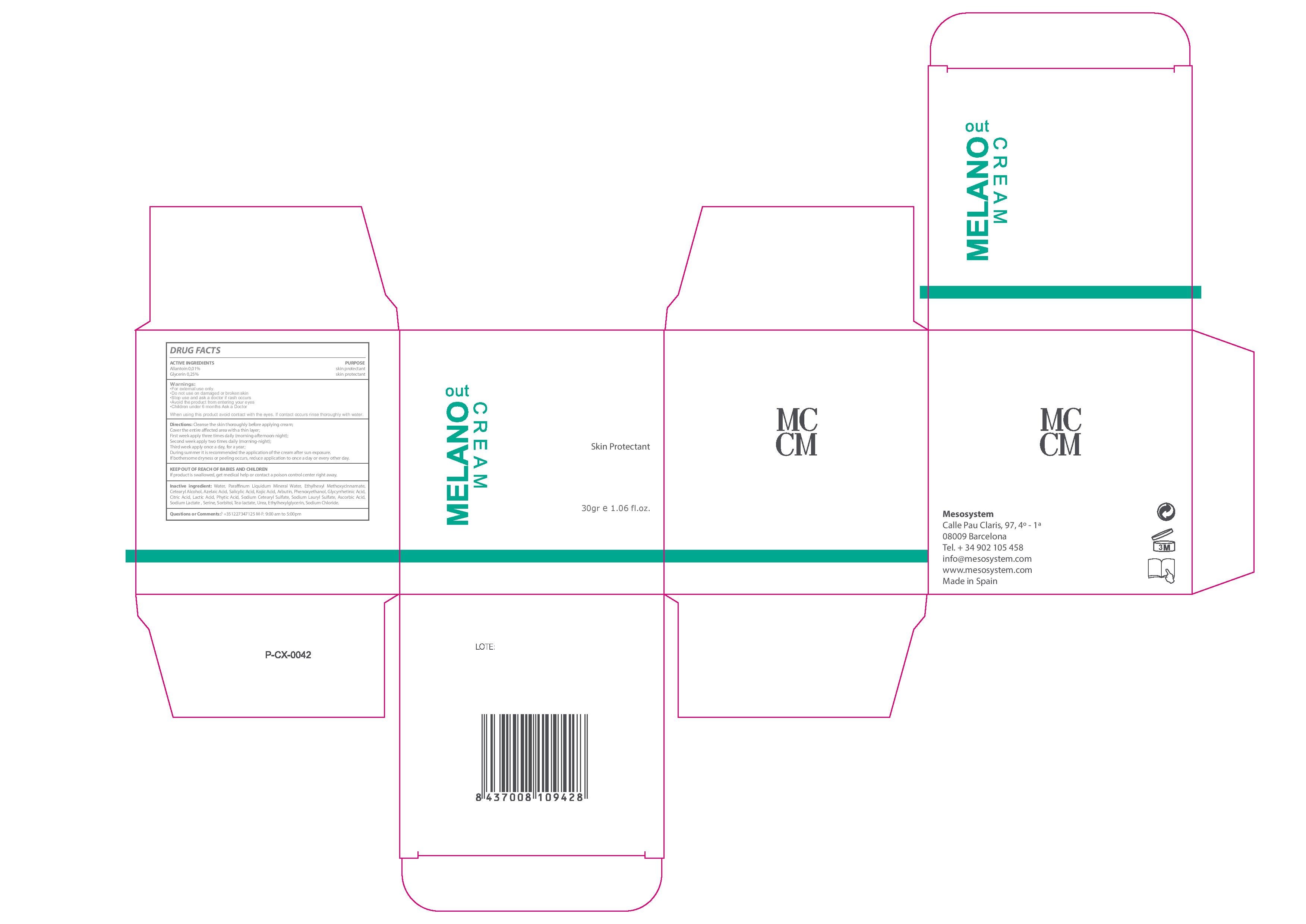
Melano Out Cream
Dosage form: cream
Ingredients: ALLANTOIN 0.1mg in 1mL, GLYCERIN 0.25mg in 1mL
Labeler: MESO SYSTEM S.A.
NDC code: 70663-007
Medically reviewed by Drugs.com. Last updated on Dec 7, 2023.
Stop use and ask a Doctor if rash occurs
- For external use only.
- Do no use on damaged or broken skin
- Stop use and ask a doctor if rash occurs
- Avoid the product from entering your eyes
- Children under 6 months Ask a Doctor
- Cream formulated to atenuate and eliminate the coetaneus spot of melanic origin located at the epidermic level
- Keep out of the reach of chuldren.
- In case of overdose get medical help or Contact a Poison Control Center right away.
- Clean the skin thoroughly before applying the cream.
- Cover the entire affected area with a thin layer. First week apply three times daily (morning-afternoon-night). Second week apply two times daily (morning-night). Third week apply once a day for one year.
- During summer it is recommended the application of the cream after sun exposure. If bothersome dryness or peeling occurs, reduce application to once a day or every other day
- +35 1227347125 M-F: 9:00 am to 5:00 pm
Active Ingredients Purpose
Allantoin 0.01%................................Skin Protectant
Glycerin 0.25%.................................Skin Protectant
Water, Azelaic Acid, Mineral Oil, Ethylhexyl Methoxycinnamate, Cetearyl Alcohol, Salicylic Acid, Kojic Acid,,Arbutin, Phenoxyethanol, Glycyrrhetinic Acid, Citric Acid, Lactic Acid, Phytic Acid, Sodium Cetearyl Sulfate, Sodium Lauryl Sulfate, Ascorbic Acid, Sodium, Lactate, Serine, Sorbitol, Tea-lactate, Urea, Ethylhexylglycerin, Sodium Chloride
- Cream formulated to atenuate and eliminate the coetaneus spot of melanic origin located at the epidermic level
| MELANO OUT CREAM
allantoin, glycerin cream |
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - MESO SYSTEM S.A. (768263100) |
| Registrant - MESO SYSTEM S.A. (768263100) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| MESO SYSTEM S.A. | 768263100 | manufacture(70663-007) | |
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.