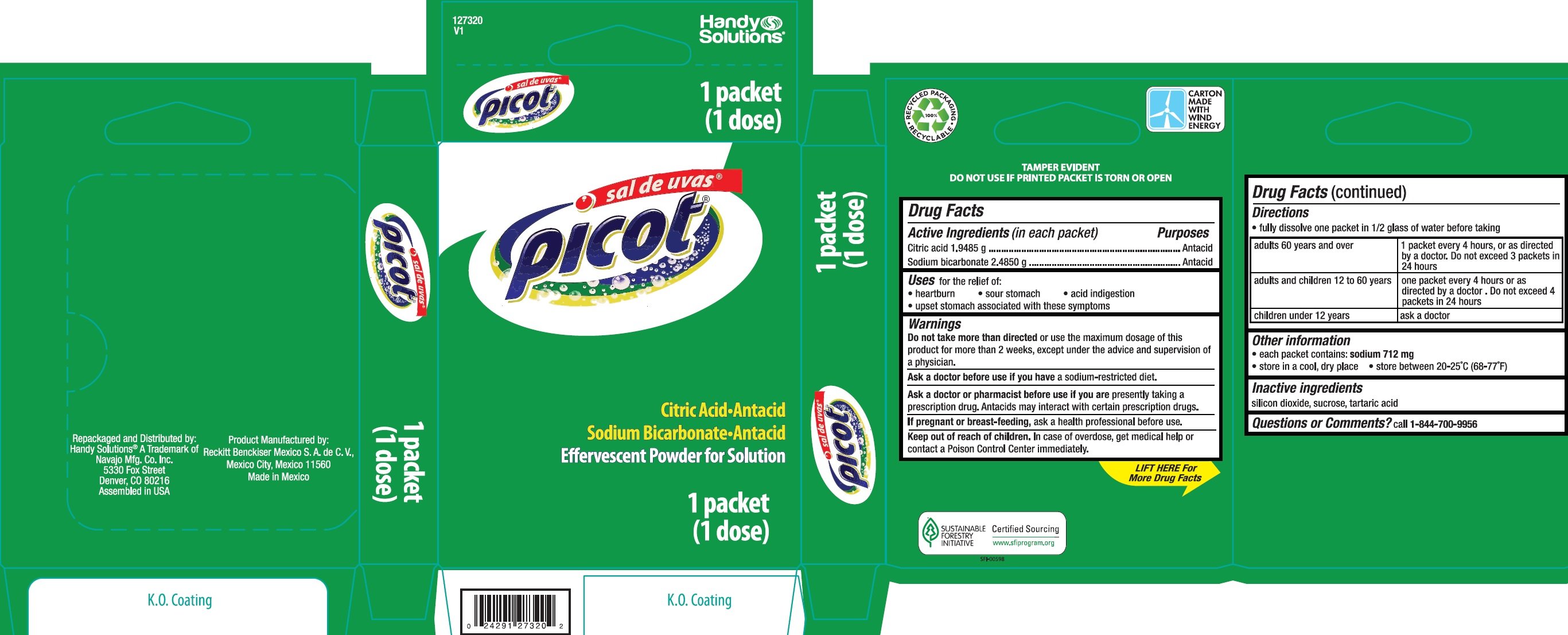
Sal De Uvas Picot
Dosage form: granule, effervescent
Ingredients: CITRIC ACID MONOHYDRATE 1.9485g, SODIUM BICARBONATE 2.485g
Labeler: Navajo Manufacturing Company Inc.
NDC code: 67751-174
Medically reviewed by Drugs.com. Last updated on Nov 13, 2023.
Citric acid 1.9485 g
Sodium bicarbonate 2.4850 g
Antacid
for the relief of:
• heartburn • sour stomach • acid indigestion
• upset stomach associated with these symptoms
more than directed or use the maximum dosage of this product for more than 2 weeks, except under the advice and supervision of a physician.
a sodium-restricted diet.
are presently taking a prescription drug. Antacids may interact with certain prescription drugs.
ask a health professional before use.
In case of overdose, get medical help or contact a Poison Control Center immediately.
- fully dissolve one packet in 1/2 glass of water before taking
| adults 60 years and over | 1 packet every 4 hours, or as directed by a doctor. Do not exceed 3 packets in 24 hours |
| adults and children 12 to 60 years | one packet every 4 hours or as directed by a doctor. Do not exceed 4 packets in 24 hours |
| children under 12 years | ask a doctor |
- each packet contains: sodium 712 mg
- store in a cool, dry place
- store between 20-25°C (68-77°F)
silicon dioxide, sucrose, tartaric acid
call 1-844-700-9956
| SAL DE UVAS PICOT
citric acid monohydrate, sodium bicarbonate granule, effervescent |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Labeler - Navajo Manufacturing Company Inc. (091917799) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Navajo Manufacturing Company Inc. | 136941411 | relabel(67751-174), repack(67751-174) | |
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.