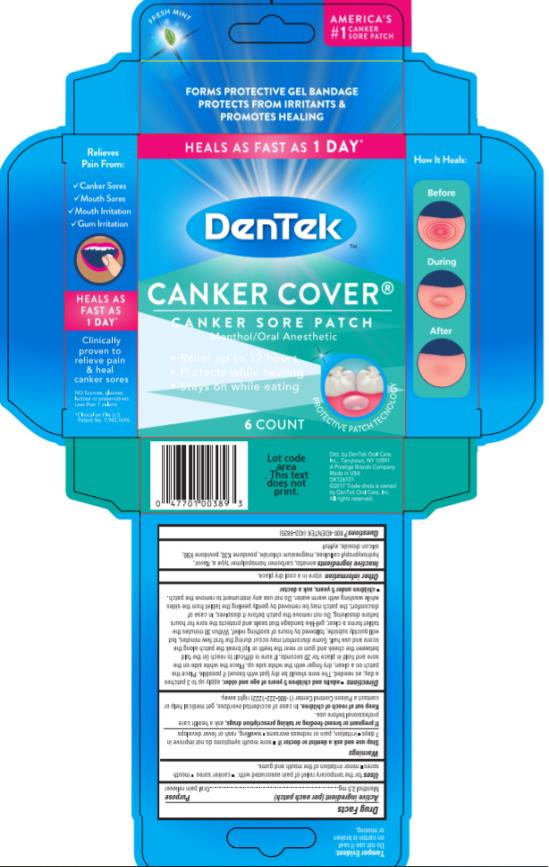
DenTek Canker Cover
Dosage form: patch, extended release
Ingredients: MENTHOL 2.5mg in 194.85mg
Labeler: DenTek Oral Care, Inc.
NDC code: 60630-091
Medically reviewed by Drugs.com. Last updated on Jun 12, 2023.
Menthol 2.5 mg
Oral pain reliever
for the temporary relief of pain associated with:
- canker sores
- mouth sores
- minor irritation of the mouth and gums.
- sore mouth symptoms do not improve in 7 days
- irritation, pain or redness worsens
- swelling, rash or fever develops
ask a health care professional before use.
In case of accidental overdose, get medical help or contact a Poison Control Center (1-800-222-1222) right away.
-
adults and children 5 years of age and older, apply up to 3 times a day, as needed
The sore should be dry (pat with tissue) if possible. Place the patch on a clean, dry finger with the white side up. Place the white side on the sore and hold in place for 20 seconds. If sore is difficult to reach (in the fold between the cheek and gum or near the teeth or lip) break the patch along the score and use half. Some discomfort may occur during the first few minutes, but will quickly subside, followed by hours of soothing relief. Within 30 minutes the tablet forms a clear, gel-like bandage that seals and protects the sore for hours before dissolving. Do not remove the patch before it dissolves. In case of discomfort, the patch may be removed by gently peeling the tablet from the sides while washing with warm water. Do not use any instrument to remove the patch.
- children under 5 years, ask a doctor
store in a cool dry place.
annatto, carbomer homopolymer type a, flavor, hydroxypropyl cellulose, potassium magnesium chloride hydrate, povidone K30, povidone K90, silicon dioxide, xylitol
800-4DENTEK (433-6835)
| DENTEK CANKER COVER
menthol patch, extended release |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - DenTek Oral Care, Inc. (153818646) |
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.