Barrier Protectant Cream
Dosage form: ointment
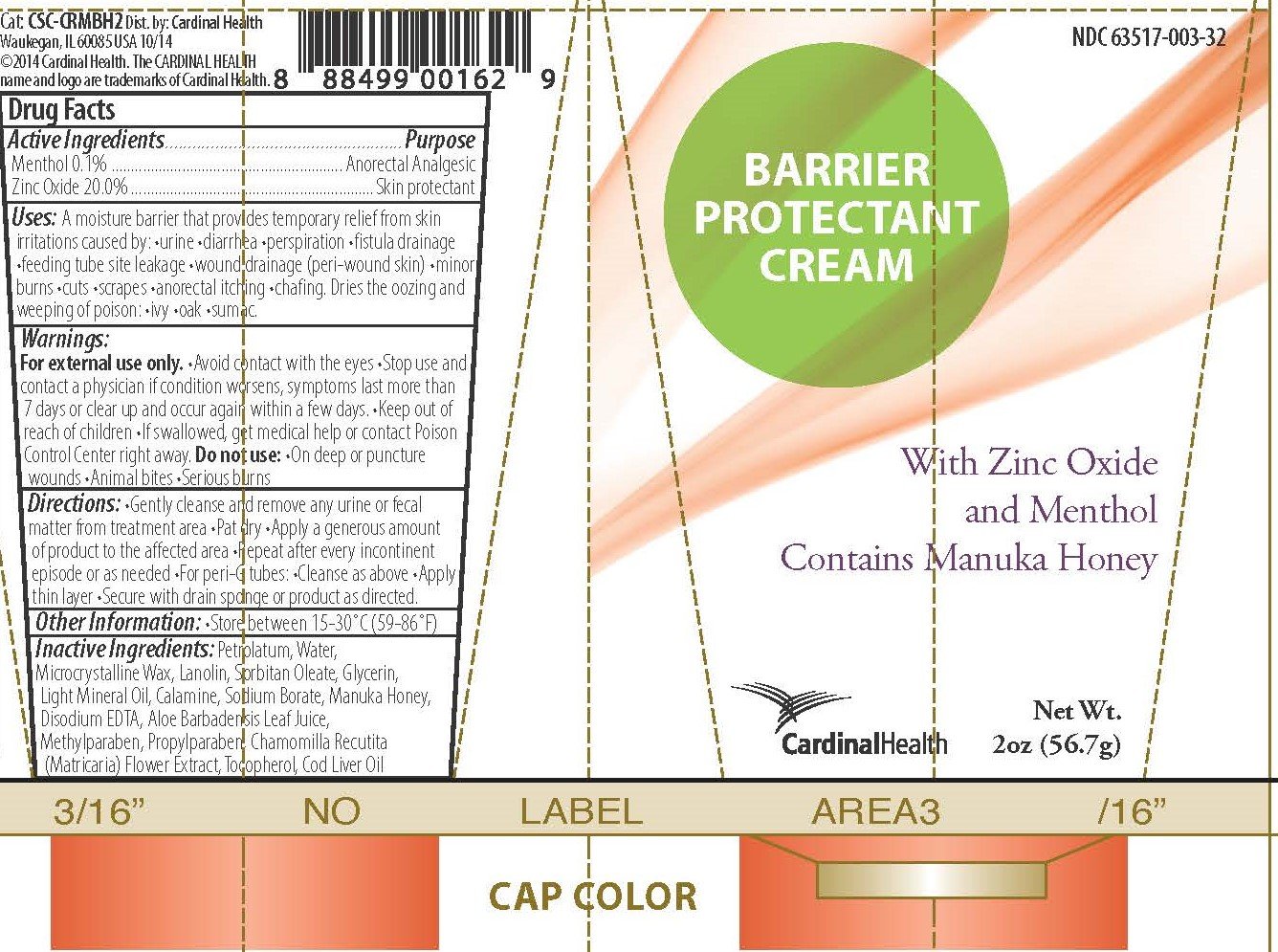
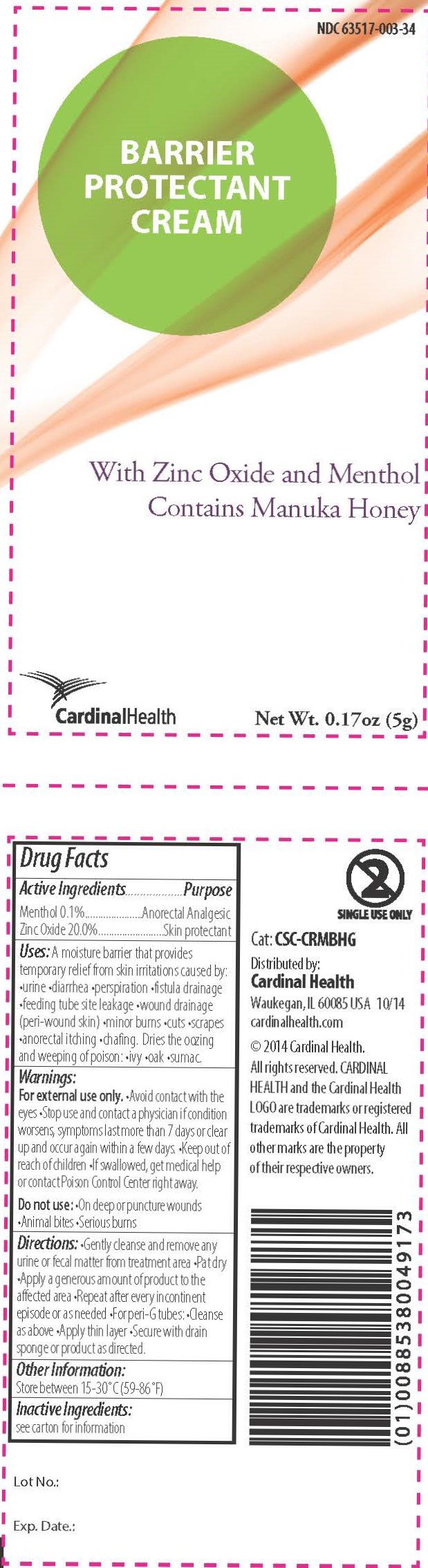
Ingredients: MENTHOL 1mg in 1g, ZINC OXIDE 200mg in 1g
Labeler: Cardinal Health
NDC code: 63517-003
Medically reviewed by Drugs.com. Last updated on Jan 16, 2024.
- Menthol 0.1%
- Zinc oxide 20%
- Anorectal analgesic
- Skin protectant
A moisture barrier that provides temporary relief from skin irritations caused by •urine •diarrhea •perspiration •fistula drainage •feeding tube site leakage •wound drainage (peri-wound skin) •minor burns •cuts •scrapes •anorectal itching •chafing
Dries the oozing and weeping of poison •ivy •oak •sumac
For external use only.
- Avoid contact with the eyes
- Stop use and contact a physician if condition worsens, symptoms last more than 7 days, or clear up and occur again within a few days.
- Keep out of reach of children
- If swallowed, get medical help or contact Poison Control Center right away.
Do not use:
- On deep or puncture wounds
- Animal bites
- Serious burns
Keep out of reach of children.
- Gently cleanse and remove any urine or fecal matter from the treatment area
- Pat dry
- Apply a generous amount of product to the affected area
- Repeat after every incontinent episode or as needed
- For peri-G tubes: •Cleanse as above •Apply thin layer •Secure with drain sponge or product as directed.
Store between 59°-86°F (15°-30°C)
Active aloe gel, Calamine, Chamomilla recutita (matricaria) flower extract, Cod liver oil, Disodium EDTA, Glycerin, Lanolin, Light mineral oil, Manuka honey, Methylparaben, Microcrystalline wax, Petrolatum, Propylparaben, Sodium borate, Sorbitan oleate, Tocopherol, Water
| BARRIER PROTECTANT CREAM
barrier cream ointment |
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
| Labeler - Cardinal Health (961027315) |
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.