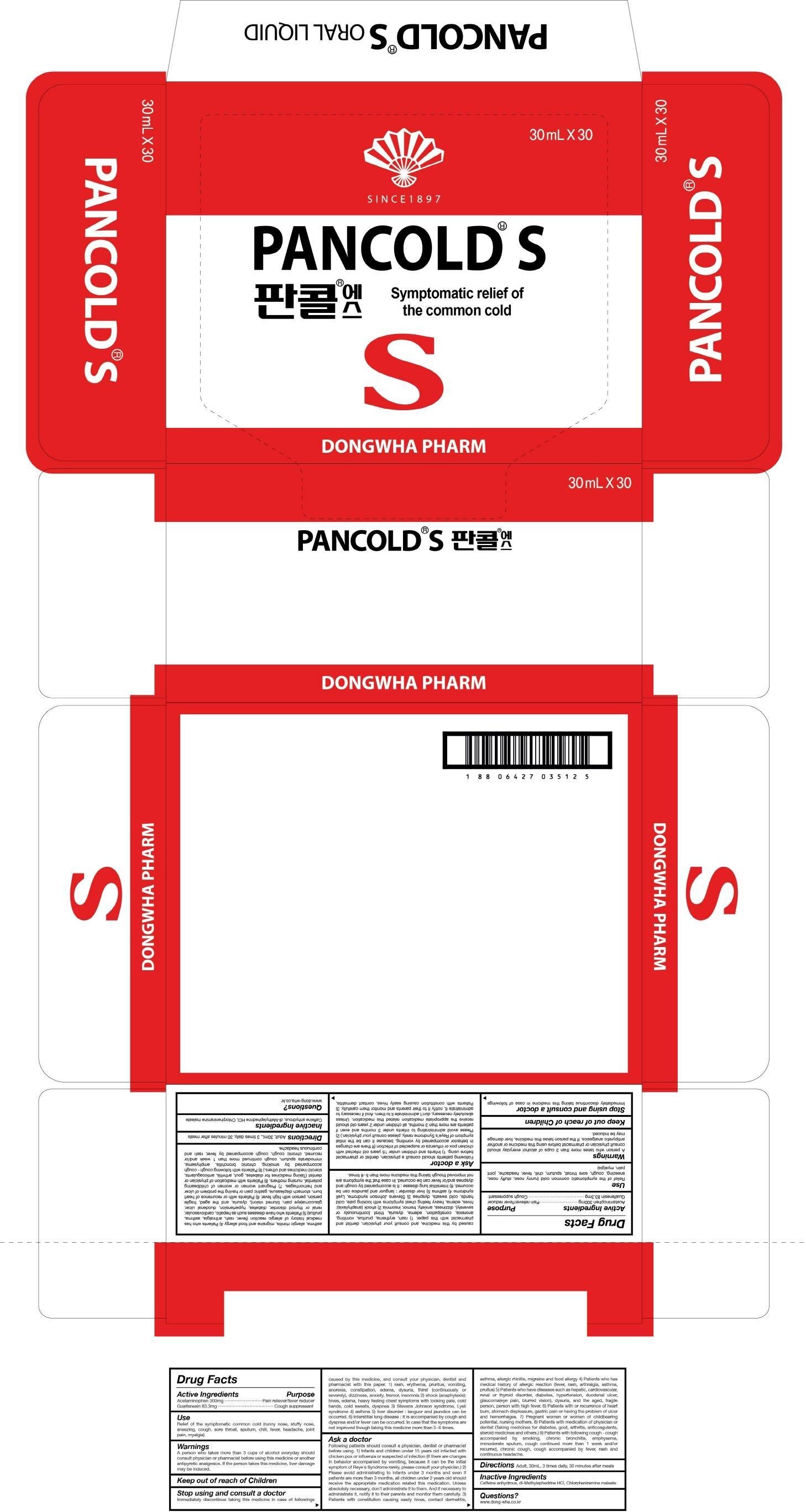
Pancold S Oral
Dosage form: liquid
Ingredients: Acetaminophen 9000mg, Guaifenesin 2499mg
Labeler: Dong Wha Pharm. Co., Ltd.
NDC code: 51352-010
Medically reviewed by Drugs.com. Last updated on Feb 15, 2024.
Active Ingredients 1 bottle contains: Acetaminophen 300mg, Guaifenesin 83.3mg
Inactive Ingredients: Caffeine anhydrous, dl-Methylephedrine HCl, Chlorpheniramine maleate
Purpose: Pain reliever/fever reducer, Cough suppressant
Warnings: A person who takes more than 3 cups of alcohol everyday should consult physician or pharmacist before using this medicine or another antipyretic analgesics. If the person takes this medicine, liver damage may be induced.
KEEP OUT OF REACH OF CHILDREN
Use: Relief of the symptomatic common cold (runny nose, stuffy nose, sneezing, cough, sore throat, sputum, chill, fever, headache, joint pain, myalgia)
Directions: Adult, 30mL, 3 times daily, 30 minutes after meals
Stop using and consult a doctor: Immediately discontinue taking this medicine in case of followings caused by this medicine, and consult your physician, dentist and pharmacist with this paper. 1) rash, erythema, pruritus, vomiting, anorexia, constipation, edema, dysuria, thirst (continuously or severely), dizziness, anxiety, tremor, insomnia 2) shock(anaphylaxis): hives, edema, heavy feeling chest symptoms with looking pale, cold hands, cold sweats, dyspnea 3) Stevens Johnson syndrome, Lyell syndrome 4) asthma 5) liver disorder : languor and jaundice can be occurred. 6) Interstitial lung disease : It is accompanied by cough and dyspnea and/or fever can be occurred. In case that the symptoms are not improved though taking this medicine more than 5-6 times.
Ask a doctor: Following patients should consult a physician, dentist or pharmacist before using. 1) Infants and children under 15 years old infected with chicken pox or influenza or suspected of infection (If there are changes in behavior accompanied by vomiting, because it can be the initial symptom of Reye's Syndrome rarely, please consult your physician.) 2) Please avoid administrating to infants under 3 months and even if patients are more than 3 months, all children under 2 years old should receive the appropriate medication related this medication. Unless absolutely necessary, don't administrate it to them. And if necessary to administrate it, notify it to their parents and monitor them carefully. 3) Patients with constitution causing easily hives, contact dermatitis, asthma, allergic rhinitis, migraine and food allergy 4) Patients who has medical history of allergic reaction (fever, rash, arthralgia, asthma, pruitus) 5) Patients who have diseases such as hepatic, cardiovascular, renal or thyroid disorder, diabetes, hypertension, duodenal ulcer, glaucoma(eye pain, blurred vision), dysuria, and the aged, fragile person, person with high fever. 6) Patients with or recurrence of heart burn, stomach displeasure, gastric pain or having the problem of ulcer and hemorrhages. 7) Pregnant women or women of childbearing potential, nursing mothers. 8) Patients with medication of physician or dentist (Taking medicines for diabetes, gout, arthritis, anticoagulants, steroid medicines and others.) 9) Patients with following cough - cough accompanied by smoking, chronic bronchitis, emphysema, immoderate sputum, cough continued more than 1 week and/or recurred, chronic cough, cough accompanied by fever, rash and continuous headache.
| PANCOLD S ORAL
acetaminophen, guaifenesin liquid |
||||||||||||
|
||||||||||||
|
||||||||||||
|
||||||||||||
|
||||||||||||
|
||||||||||||
| Labeler - Dong Wha Pharm. Co., Ltd. (687745240) |
| Registrant - Dong Wha Pharm. Co., Ltd. (687745240) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Dong Wha Pharm. Co., Ltd. | 687745240 | manufacture(51352-010) | |
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.