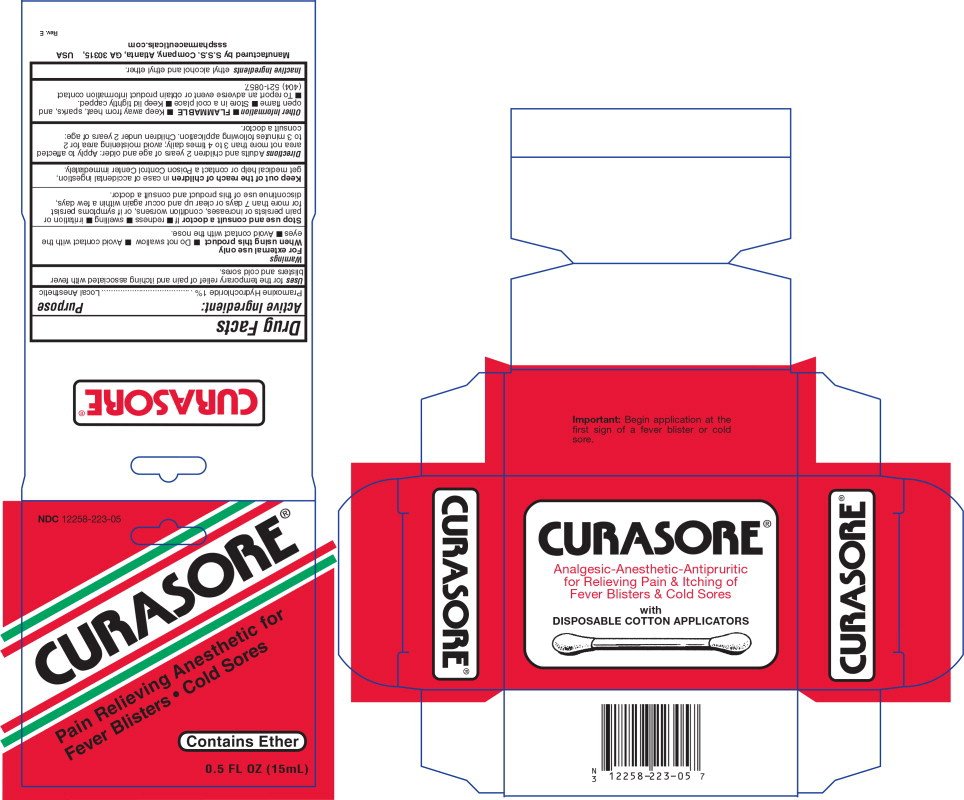
Curasore
Dosage form: liquid
Ingredients: PRAMOXINE HYDROCHLORIDE 1g in 100mL
Labeler: S.S.S. Company
NDC code: 12258-223
Medically reviewed by Drugs.com. Last updated on Sep 21, 2023.
DRUG FACTS
Pramoxine Hydrochloride 1%
Local Anesthetic
For the temporary relief of pain and itching associated with fever blisters and cold sores
For external use only
- Do not swallow
- Avoid contact with the eyes
- Avoid contact with the nose
Stop use and consult a doctor If
- Redness
- Swelling
- Irritation or pain persists or increases, condition worsens, or if symptoms persist for more than 7 days or clear up and occur again within a few days, discontinue use of this product and consult a doctor.
In case of accidental ingestion, get medical help or contact a Poison Control Center immediately.
Adults and children 2 years of age and older: Apply to affected area not more than 3 to 4 times daily; avoid moistening area for 2 to 3 minutes following application. Children under 2 years of age: consult a doctor.
- FLAMMABLE
- Keep away from heat, sparks, and open flame
- Store in a cool place
- Keep lid tightly capped.
- To report an adverse event or obtain product information contact (404) 521-0857.
ethyl alcohol and ethyl ether
NDC 12258-223-05
CURASORE®
Pain Relieving Anesthetic for
Fever Blisters • Cold Sores
Contains Ether
0.5 FL OZ (15mL)
Important: Begin application at the
first sign of a fever blister or cold
sore.
CURASORE®
Analgesic-Anesthetic-Antipruritic
for Relieving Pain & Itching of
Fever Blisters & Cold Sores
With
DISPOSABLE COTTON APPLICATORS
Other Packaging Content
Manufactured by S.S.S. Company, Atlanta, GA 30315, USA
ssspharmaceuticals.com
Rev. E
| CURASORE
pramoxine hydrochloride liquid |
|||||||||||||
|
|||||||||||||
|
|||||||||||||
|
|||||||||||||
|
|||||||||||||
|
|||||||||||||
| Labeler - S.S.S. Company (003288321) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| S.S.S. Company | 003288321 | manufacture(12258-223), pack(12258-223), label(12258-223) | |
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.