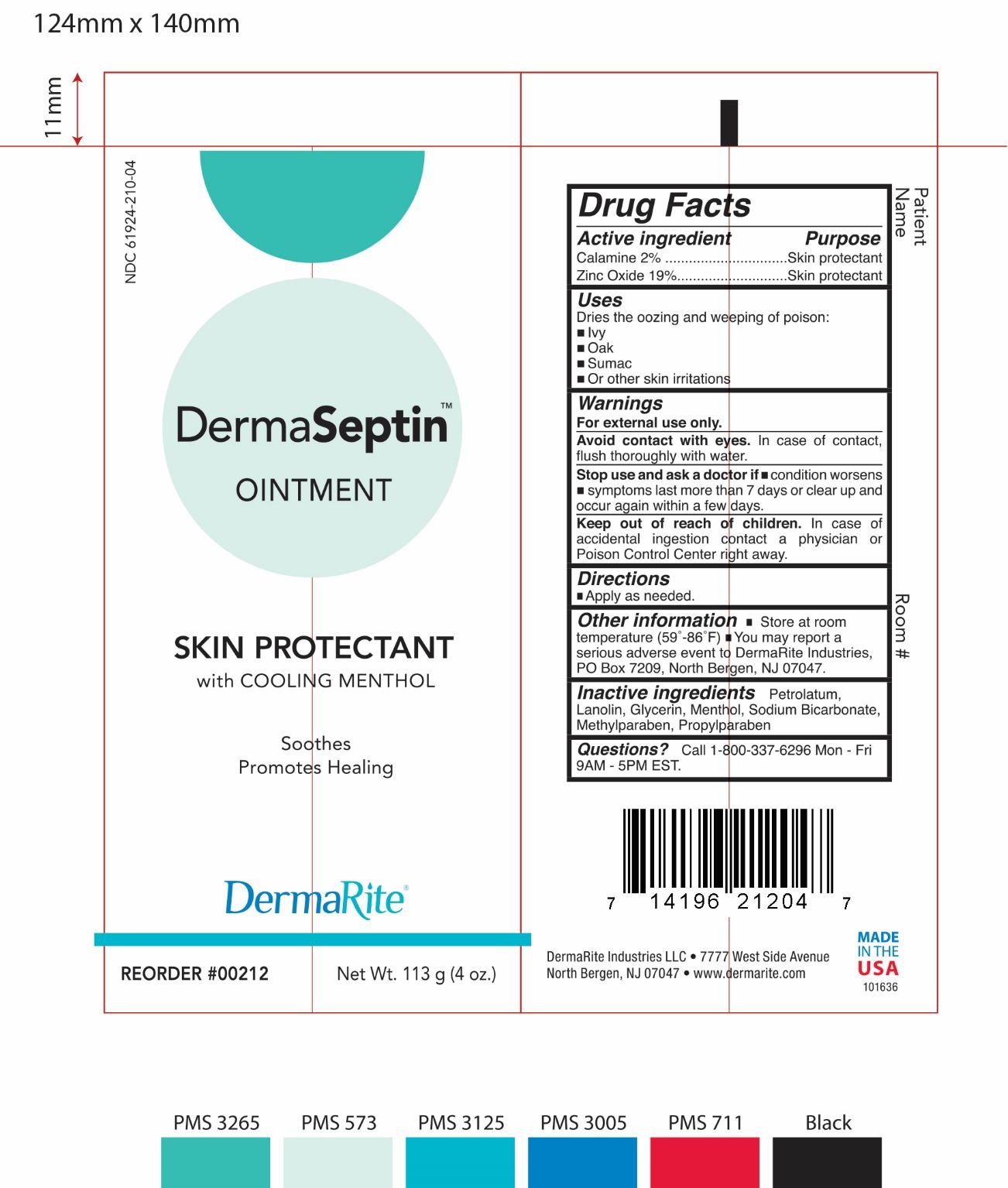
DERMASEPTIN
Dosage form: ointment
Ingredients: FERRIC OXIDE RED 2.3g in 1g, ZINC OXIDE 21.47g in 1g
Labeler: DermaRite Industries, LLC
NDC code: 61924-210
Medically reviewed by Drugs.com. Last updated on Sep 4, 2023.
Active Ingredients
- Zinc Oxide 19%
- Calamine 2%
Purpose:
- Zinc Oxide: Skin protectant
- Calamine : Skin protectant
Uses:
Dries the oozing and weeping of poison:
- Ivy
- Oak
- Sumac
- Or othe skin irritations
Warnings
- For external use only.
- Avoid contact with eyes. In case of contact, flush thoroughly with water.
- Stop use and ask a doctor if condition worsens
- Symptoms last more than 7 days or clear up and occur again within a few days.
Warnings:
- Keep out of reach of children. In case accidental ingestion contact a physician or Poison Control Center right away.
Directions
Applied as needed.
Other Information:
Store at room temperature (59°-86°F).
You may report a serious adverse event to DermaRite Industires, PO Box 7209, North Bergen, NJ 07047.
Inactive Ingredients:
Petrolatum, Lanolin, Glycerin, Menthol, Sodium Bicarbonate, methylparaben, Propylparaben
Questions
Call 1-800-337-6296 Mon-Fri 9AM-5PM EST.
| DERMASEPTIN
otc skin protectant drug products ointment |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Labeler - DermaRite Industries, LLC (883925562) |
| Registrant - DermaRite Industries, LLC (883925562) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| DermaRite Industries, LLC | 883925562 | manufacture(61924-210) | |
Document Id: 75c6aee4-84cc-55df-e053-2991aa0ae7d1
Set id: 0e75b4ea-4dee-4216-94f1-5b273f3c6911
Version: 4
Effective Time: 20180913
DermaRite Industries, LLC
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.