62% Ethyl Alcohol Antiseptic Nasal
Dosage form: swab
Ingredients: ALCOHOL 62mL in 100mL
Labeler: Medline Industries, Inc.
NDC code: 53329-132
Medically reviewed by Drugs.com. Last updated on Apr 8, 2024.
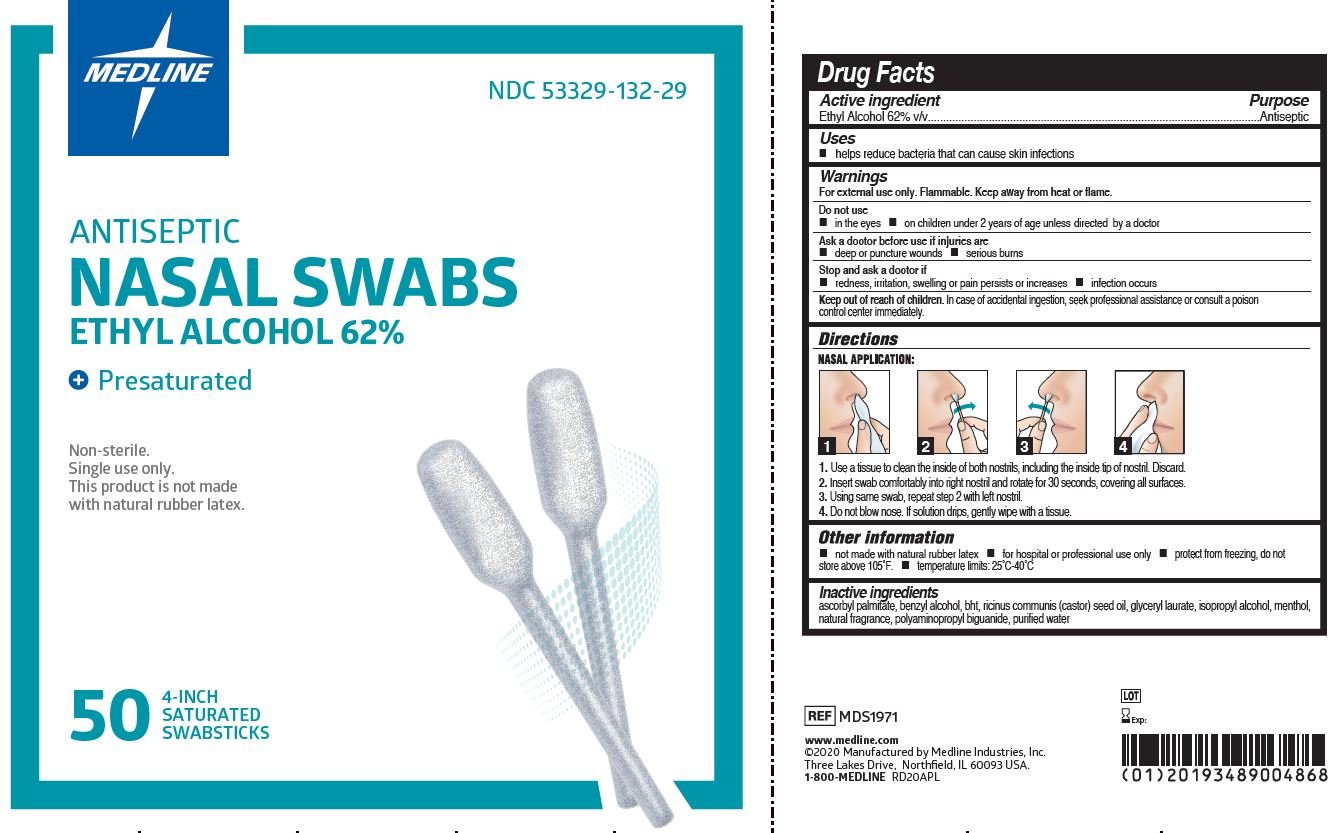
Ethyl Alcohol 62% v/v
Antiseptic
- helps reduce bacteria that can cause skin infections
For external use only. Flammable. Keep away from heat or flame.
- in the eyes
- on children under 2 years of age unless directed by a doctor
- deep or puncture wounds
- serious burns
- redness, irritation, swelling or pain persists or increases
- infection occurs
In case of accidental ingestion, seek professional assistance or consult a poison control center immediately.
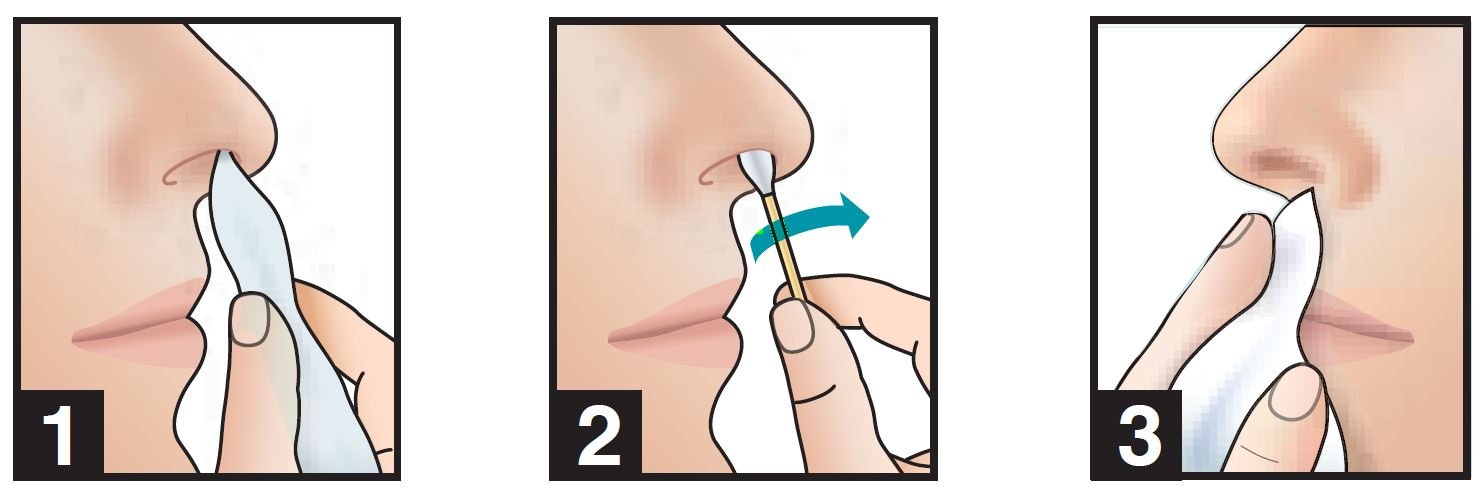
1. Use a tissue to clean the inside of both nostrils, including the inside tip of nostril. Discard.
2. Insert swab comfortably into right nostril and rotate for 30 seconds, covering all surfaces.
3. Using same swab, repeat step 2 with left nostril.
4. Do not blow nose. If solution drips, gently wipe with a tissue.
- not made with natural rubber latex
- for hospital or professional use only
- protect from freezing, do not store above 105ºF
- temperature limits: 25ºC-40ºC
ascorbyl palmitate, benzyl alcohol, BHT, ricinus communis (castor) seed oil, glyceryl laurate, isopropyl alcohol, menthol, natural fragrance, polyaminopropyl biguanide, purified water
| 62% ETHYL ALCOHOL ANTISEPTIC NASAL
ethyl alcohol swab |
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
| Labeler - Medline Industries, Inc. (025460908) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Aplicare Products, LLC | 081054904 | manufacture(53329-132) | |
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.