G SOL
Dosage form: spray
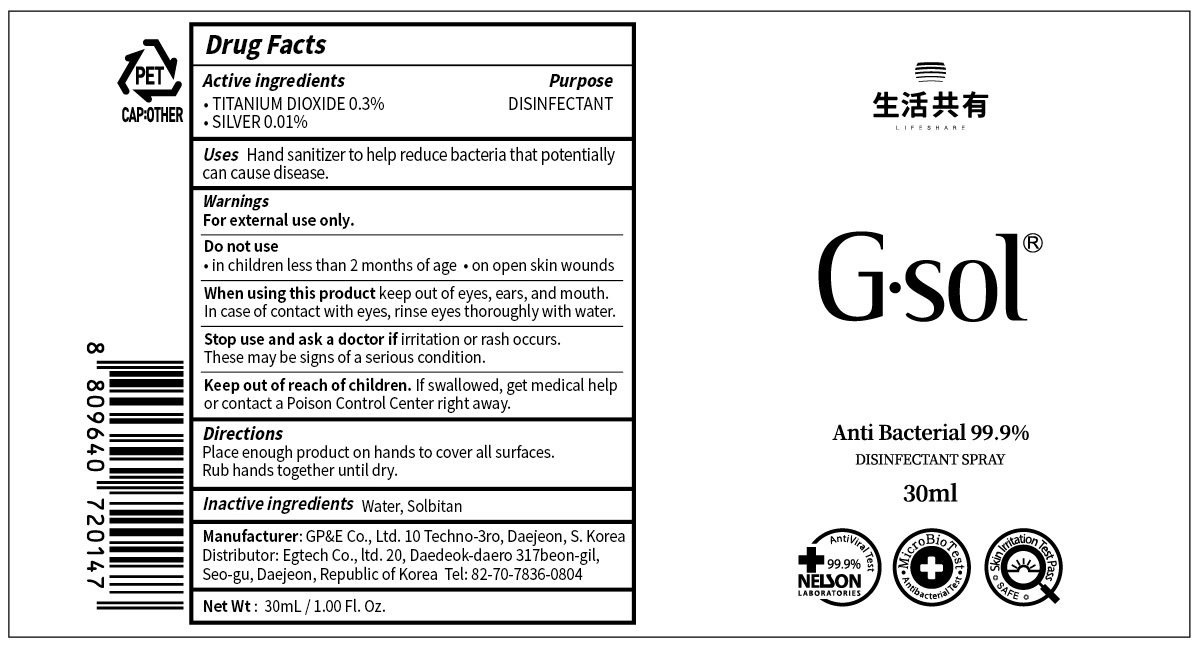
Ingredients: TITANIUM DIOXIDE 0.3g in 100mL, SILVER 0.01g in 100mL
Labeler: Egtech co., Ltd.
NDC code: 76767-020
Medically reviewed by Drugs.com. Last updated on May 9, 2024.
TITANIUM DIOXIDE 0.3%
SILVER 0.01%
Water, Sorbitan
DISINFECTANT
For external use only.
--------------------------------------------------------------------------------------------------------
Do not use
• in children less than 2 months of age
• on open skin wounds
--------------------------------------------------------------------------------------------------------
When using this product keep out of eyes, ears, and mouth. In case of contact with eyes, rinse eyes thoroughly with water.
--------------------------------------------------------------------------------------------------------
Stop use and ask a doctor if irritation or rash occurs. These may be signs of a serious condition.
If swallowed, get medical help or contact a Poison Control Center right away.
Hand sanitizer to help reduce bacteria that potentially can cause disease.
Place enough product on hands to cover all surfaces. Rub hands together until dry.
| G SOL
titanium dioxide, silver spray |
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
| Labeler - Egtech co., Ltd. (690074770) |
| Registrant - Egtech co., Ltd. (690074770) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| GP&E Co., Ltd | 689816478 | manufacture(76767-020) | |
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.