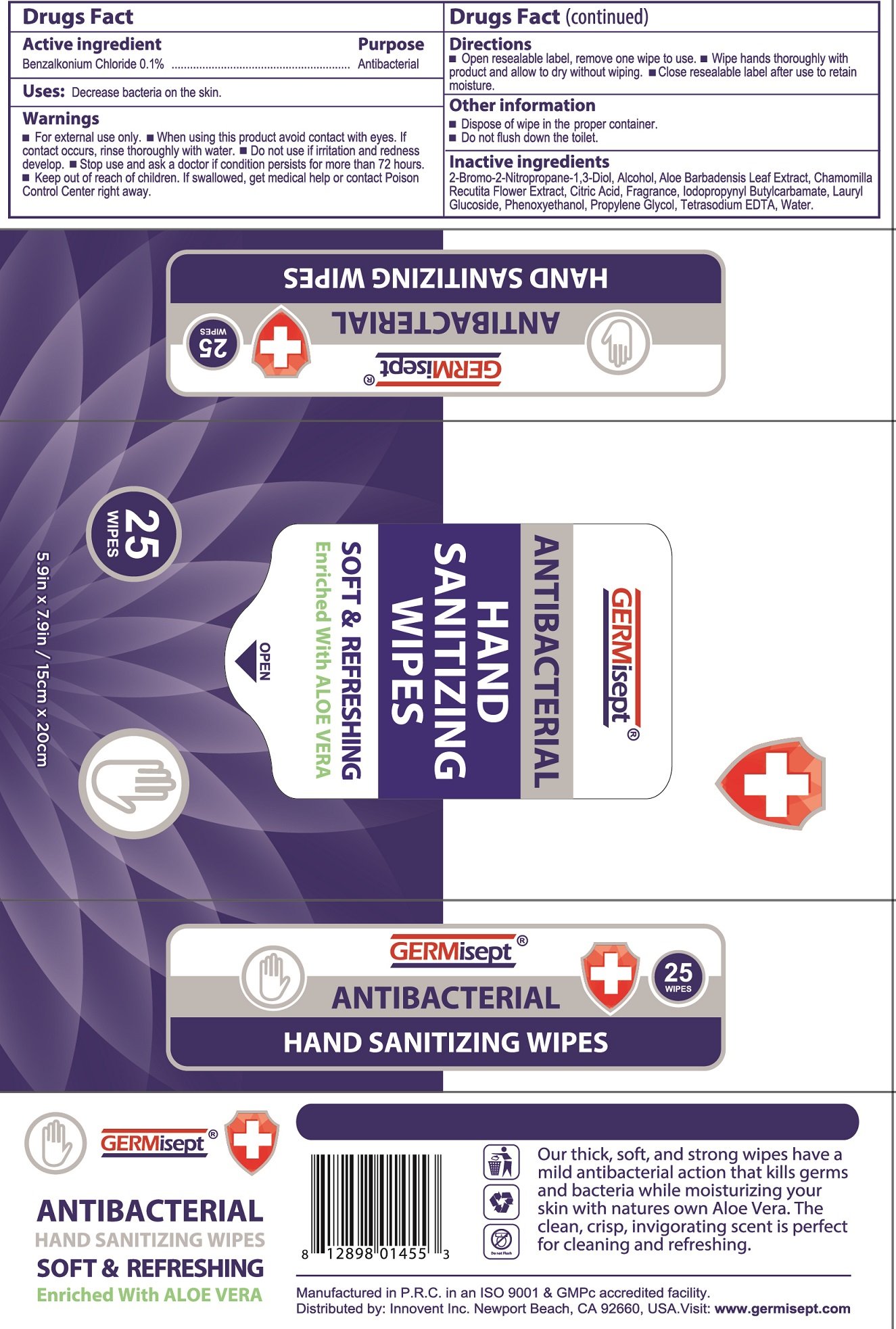
Germisept Antibacterial Hand Sanitizing Wipes
Dosage form: cloth
Ingredients: BENZALKONIUM CHLORIDE 1mg in 1mL
Labeler: Innovent Inc
NDC code: 70335-304
Medically reviewed by Drugs.com. Last updated on Aug 21, 2023.
Drugs Fact
Active ingredient
Benzalkonium Chloride 0.1%
Purpose
Antibacterial
Uses:
Decrease bacteria on the skin.
Warnings
- For external use only.
When using this product
- avoid contact with eyes. If contact occurs, rinse thorughly with water.
Do not use
- if irritation and redness develop.
Stop use and ask a doctor
- if condition persists for more than 72 hours.
Keep out of reach of children.
- If swallowed, get medical help or contact Poison Control Center right away.
Directions
- Open resealable label, remove one wipe to use.
- Wipe hands thoroughly with product and allow to dry without wiping.
- Close resealable label after use to retain moisture.
Other information
- Dispose of wipe in the proper container.
- Do not flush down the toilet.
Inactive ingredients
2-Bromo-2-Nitropropane-1,3-Diol, Alcohol, Aloe Barbadensis Leaf Extract, Chamomilla Recutita Flower Extract, Citric Acid, Fragrance, Iodopropynyl Butylcarbamate, Lauryl Glucoside, Phenoxyethanol, Propylene Glycol, Tetrasodium EDTA, Water.
| GERMISEPT ANTIBACTERIAL HAND SANITIZING WIPES
benzalkonium chloride cloth |
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| Labeler - Innovent Inc (079973489) |
Document Id: ae2c50b5-0a16-84ef-e053-2995a90ac360
Set id: f5f90e47-6783-4942-8797-4a28346f25c0
Version: 5
Effective Time: 20200831
Innovent Inc
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.