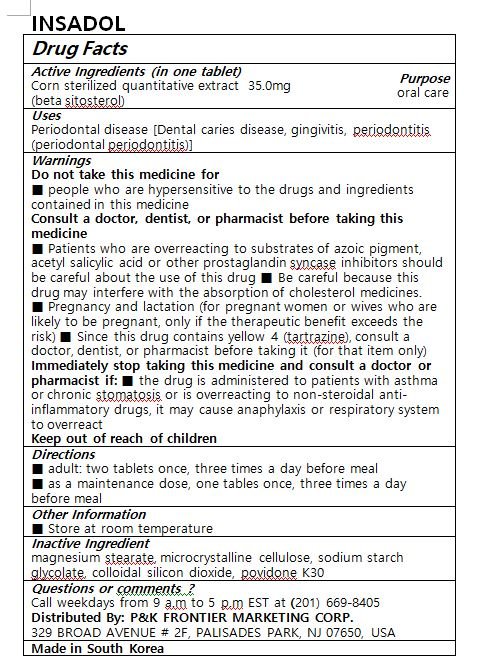
INSADOL
Dosage form: tablet
Ingredients: .BETA.-SITOSTEROL 35mg
Labeler: I World Pharmaceutical Co., Ltd.
NDC code: 73442-0002
Medically reviewed by Drugs.com. Last updated on Oct 30, 2023.
Corn sterilized quantitative extract (beta sitosterol)
Periodontal disease [Dental caries disease, gingivitis, periodontitis (periodontal periodontitis)]
Keep out of reach of children
■ adult: two tablets once, three times a day before meal
■ as a maintenance dose, one tablet once, three times a day before meal
Do not take this medicine if
■ people who are hypersensitive to the drugs and ingredients contained in this medicine
Consult a doctor, dentist, or pharmacist before taking this medicine
■ Patients who are overreacting to substrates of azoic pigment, acetyl salicylic acid or other prostaglandin syncase inhibitors should be careful about the use of this drug ■ Be careful because this drug may interfere with the absorption of cholesterol medicines.
■ Pregnancy and lactation (for pregnant women or wives who are likely to be pregnant, only if the therapeutic benefit exceeds the risk) ■ Since this drug contains yellow 4 (tartrazine), consult a doctor, dentist, or pharmacist before taking it (for that item only)
Immediately stop taking this medicine and consult a doctor or pharmacist if: ■ the drug is administered to patients with asthma or chronic stomatosis or is overreacting to non-steroidal anti-inflammatory drugs, it may cause anaphylaxis or respiratory system to overreact
magnesium stearate, microcrystalline cellulose, sodium starch glycolate, colloidal silicon dioxide, povidone K30
For oral use only
| INSADOL
corn sterilized quantitative extract (beta sitosterol) tablet |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Labeler - I World Pharmaceutical Co., Ltd. (688222857) |
| Registrant - I World Pharmaceutical Co., Ltd. (688222857) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| I World Pharmaceutical Co., Ltd | 688222857 | manufacture(73442-0002) | |
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.