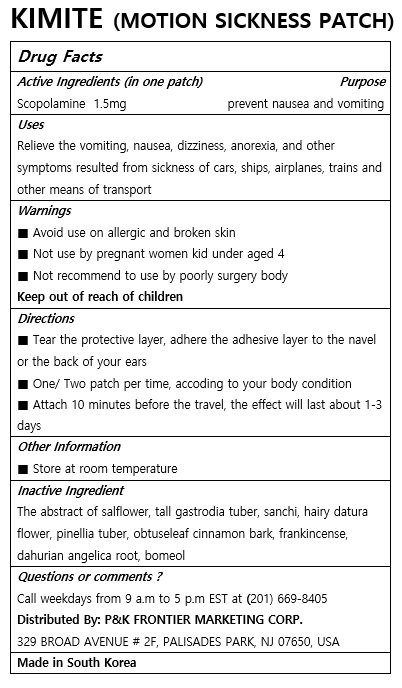
KIMITE
Dosage form: patch, extended release
Ingredients: SCOPOLAMINE 1.5mg
Labeler: OASIS TRADING
NDC code: 72689-0023
Medically reviewed by Drugs.com. Last updated on Dec 4, 2023.
Scopolamine
Relieve the vomiting, nausea, dizziness, anorexia, and other symptoms resulted from sickness of cars, ships, airplanes, trains and other means of transport
Keep out of reach of children
■ Tear the protective layer, adhere the adhesive layer to the navel or the back of your ears
■ One/ Two patch per time, accoding to your body condition
■ Attach 10 minutes before the travel, the effect will last about 1-3 days
■ Avoid use on allergic and broken skin
■ Not use by pregnant women kid under aged 4
■ Not recommend to use by poorly surgery body
The abstract of salflower, tall gastrodia tuber, sanchi, hairy datura flower, pinellia tuber, obtuseleaf cinnamon bark, frankincense, dahurian angelica root, bomeol
For external use only
| KIMITE
scopolamine patch, extended release |
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
| Labeler - OASIS TRADING (689991468) |
| Registrant - OASIS TRADING (689991468) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| OASIS TRADING | 689991468 | manufacture(72689-0023) | |
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.