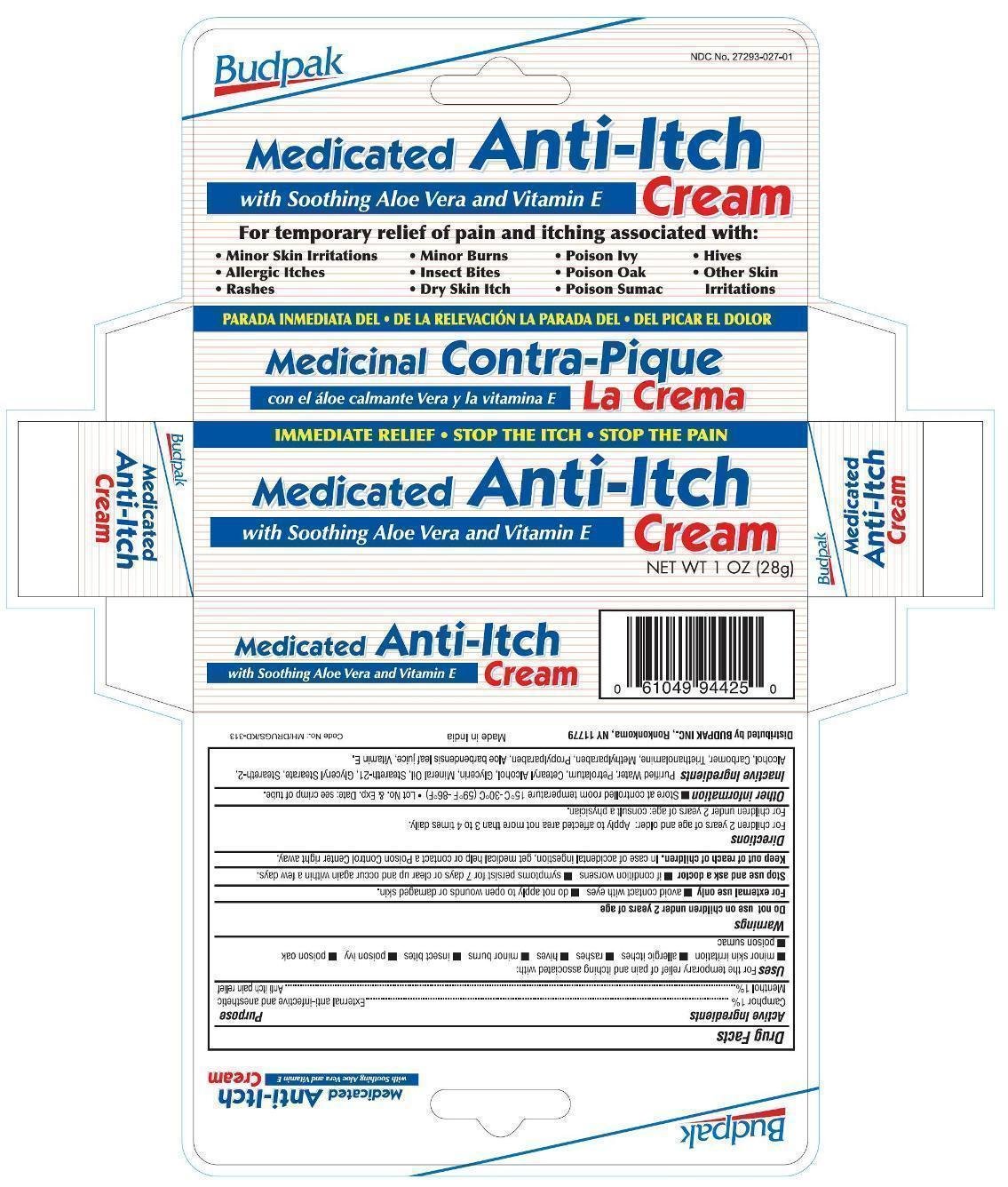
Budpak Medicated Anti Itch
Dosage form: cream
Ingredients: CAMPHOR (SYNTHETIC) 0.01g in 1g, MENTHOL 0.01g in 1g
Labeler: Budpak Inc.
NDC code: 27293-027
Medically reviewed by Drugs.com. Last updated on Dec 27, 2023.
Active Ingredients
Camphor 1%
Menthol 1%
Purpose
External anti-infective and anesthetic
Anti itch pain relief
Uses
For the temporary relief of pain and itching associated with:
- minor skin irritation
- allergic itches
- rashes
- hives
- minor burns
- insect bites
- poison ivy
- poison oak
- poison sumac
Warnings
For external use only
Do not use on children under 2 years of age.
- avoid contact with eyes
- do not apply to open wound or damaged skin.
Stop use and ask a doctor
- if condition worsens
- symptoms persist for 7 days or clear up and occur again within a few days.
Keep out of reach of children.
In case of accidental ingestion, get medical help or contact a Poison Control Center right away.
Directions
-
For children 2 years of age and older: Apply to affected area not more than 3 to 4 times daily.
-
For children under 2 years of age: consult a physician.
Other information
- Store at controlled room temperature 15ºC to 30ºC (59ºF to 86ºF)
- Lot No. & Exp. Date: see crimp of tube.
Inactive ingredients
Purified Water, Petrolatum, Cetearyl Alcohol, Glycerin, Mineral Oil, Steareth-21, Glyceryl Stearate, Steareth-2, Alcohol, Carbomer, Triethanolamine, Methylparaben, Propylparaben, Aloe barbadensis leaf juice, Vitamin E.
PRINCIPAL DISPLAY PANEL
Budpak Medicated Anti-Itch Cream
Camphor 1%
Menthol 1%
NET WT 1 OZ (28 g)
| BUDPAK MEDICATED ANTI ITCH
camphor and menthol cream |
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
| Labeler - Budpak Inc. (183224849) |
| Registrant - Anicare Pharmaceuticals Pvt. Ltd (916837425) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Anicare Pharmaceuticals Pvt. Ltd | 916837425 | manufacture(27293-027) | |
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.