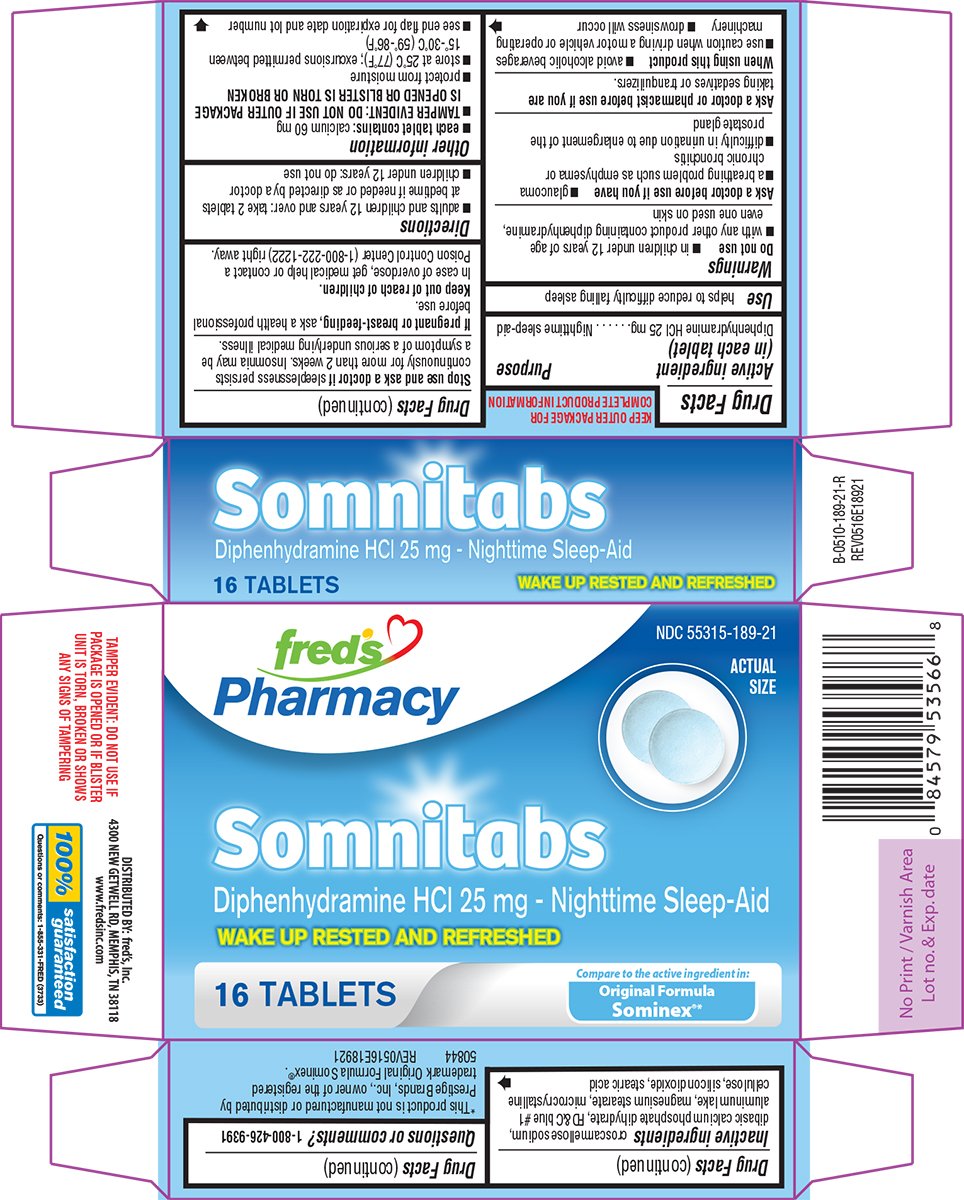
Somnitabs
Dosage form: tablet
Ingredients: DIPHENHYDRAMINE HYDROCHLORIDE 25mg
Labeler: FRED'S, INC.
NDC code: 55315-189
Medically reviewed by Drugs.com. Last updated on Jun 2, 2023.
Diphenhydramine HCl 25 mg
Nighttime sleep-aid
helps to reduce difficulty falling asleep
- in children under 12 years of age
- with any other product containing diphenhydramine, even one used on skin
- glaucoma
- a breathing problem such as emphysema or chronic bronchitis
- difficulty in urination due to enlargement of the prostate gland
taking sedatives or tranquilizers.
- avoid alcoholic beverages
- use caution when driving a motor vehicle or operating machinery
- drowsiness will occur
sleeplessness persists continuously for more than 2 weeks. Insomnia may be a symptom of a serious underlying medical illness.
ask a health professional before use.
In case of overdose, get medical help or contact a Poison Control Center (1-800-222-1222) right away.
- adults and children 12 years and over: take 2 tablets at bedtime if needed or as directed by a doctor
- children under 12 years: do not use
- each tablet contains: calcium 60 mg
- TAMPER EVIDENT: DO NOT USE IF OUTER PACKAGE IS OPENED OR BLISTER IS TORN OR BROKEN
- protect from moisture
- store at 25°C (77°F); excursions permitted between 15°-30°C (59°-86°F)
- see end flap for expiration date and lot number
croscarmellose sodium, dibasic calcium phosphate dihydrate, FD&C blue #1 aluminum lake, magnesium stearate, microcrystalline cellulose, silicon dioxide, stearic acid
fred's
Pharmacy
NDC 55315-189-21
ACTUAL
SIZE
Somnitabs
Diphenhydramine HCl 25 mg - Nighttime Sleep-Aid
WAKE UP RESTED AND REFRESHED
16 TABLETS
Compare to the active ingredient in:
Original Formula
Sominex®*
TAMPER EVIDENT: DO NOT USE IF
PACKAGE IS OPENED OR IF BLISTER
UNIT IS TORN, BROKEN OR SHOWS
ANY SIGNS OF TAMPERING
*This product is not manufactured or distributed by
Prestige Brands, Inc., owner of the registered
trademark Original Formula Sominex®.
50844 REV0516E18921
DISTRIBUTED BY: fred's, Inc.
4300 NEW GETWELL RD, MEMPHIS, TN 38118
www.fredsinc.com
100%
satisfaction
guaranteed
Questions or comments: 1-855-331-FRED (3733)
| SOMNITABS
diphenhydramine hcl tablet |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Labeler - FRED'S, INC. (005866116) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| LNK International, Inc. | 038154464 | PACK(55315-189) | |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| LNK International, Inc. | 832867894 | MANUFACTURE(55315-189) | |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| LNK International, Inc. | 967626305 | PACK(55315-189) | |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| LNK International, Inc. | 832867837 | PACK(55315-189) | |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| LNK International, Inc. | 868734088 | PACK(55315-189) | |
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.