PerioMed
Dosage form: rinse
Ingredients: stannous fluoride 1.53mg in 1g
Labeler: 3M ESPE Dental Products
NDC code: 48878-3315
Medically reviewed by Drugs.com. Last updated on Jun 2, 2023.
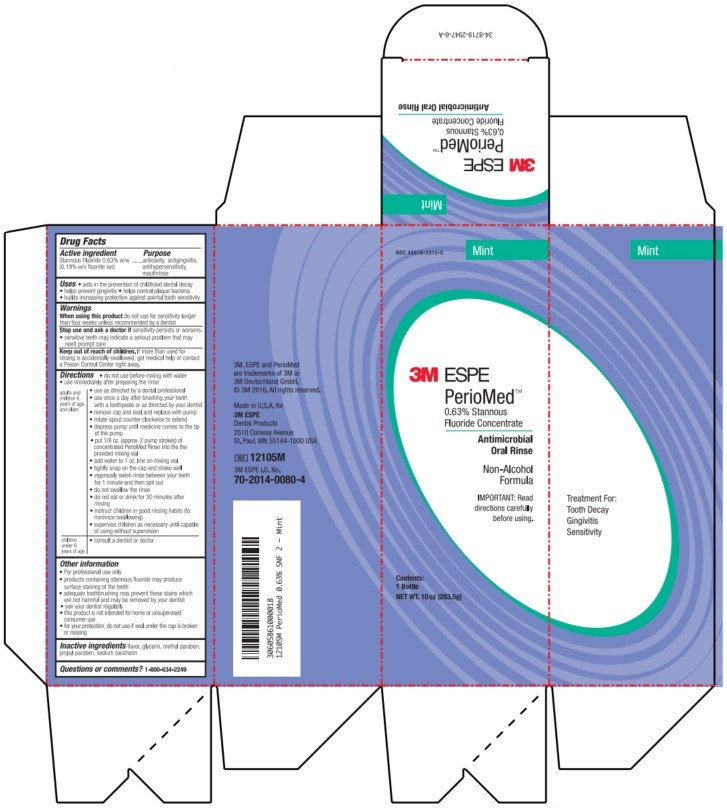
Drug Facts
Stannous fluoride 0.63% w/w (0.19% w/v flouride ion)
anticavity, antigingivitis, antihypersensitivity, mouthrinse
- aids in the prevention of childhood dental decay
- helps prevent gingivitis
- helps control plaque bacteria
- builds increasing protection against painful tooth sensitivity
- When using this product do not use for sensitivity longer than four weeks unless recommended by a dentist.
- Stop use and ask a doctor if sensitivity persists or worsens.
- Sensitive teeth may indicate a serious problem that may need prompt care.
- Keep out of reach of children. If more than used for rinsing is accidentally swallowed, get medical help or contact a Poison Control Center right away.
adults and children 6 years of age and older:
- do not use before mixing with water
- use immediately after preparing the rinse
- use as directed by a dental professional
- use once a day after brushing your teeth with a toothpaste or as directed by your dentist
- remove cap and seal and replace with pump
- rotate spout counter clockwise to extend
- depress pump until medicine comes to the tip of the pump
- put 1/8 oz. (approx. 2 pump strokes) of concentrated PerioMed Rinse into the provided mixing vial
- add water to 1 oz. line on mixing vial
- tightly snap on the cap and shake well
- vigorously swish rinse between your teeth for 1 minute and then spit out
- do not swallow the rinse
- do not eat or drink for 30 minutes after rinsing
- instruct children in good rinsing habits (to minimize swallowing)
- supervise children as necessary until capable of using without supervision
children under 6 years of age:
- consult a dentist or doctor
- For professional use only
- products containing stannous fluoride may produce surface staining of the teeth
- adequate toothbrushing may prevent these stains which are not harmful and may be removed by your dentist
- see your dentist regularly
- this product is not intended for home or unsupervised consumer use
- for your protection, do not use if seal under the cap is broken or missing
flavor, glycerin, methyl paraben, propyl paraben, sodium saccharin
NDC 48878-3315-0
Mint
3M ESPE
PerioMed™
0.63% Stannous
Fluoride Concentrate
Antimicrobial
Oral Rinse
Non-Alcohol
Formula
IMPORTANT: Read
directions carefully
before using.
Contents:
1 Bottle
NET WT. 10 oz (283.5g)
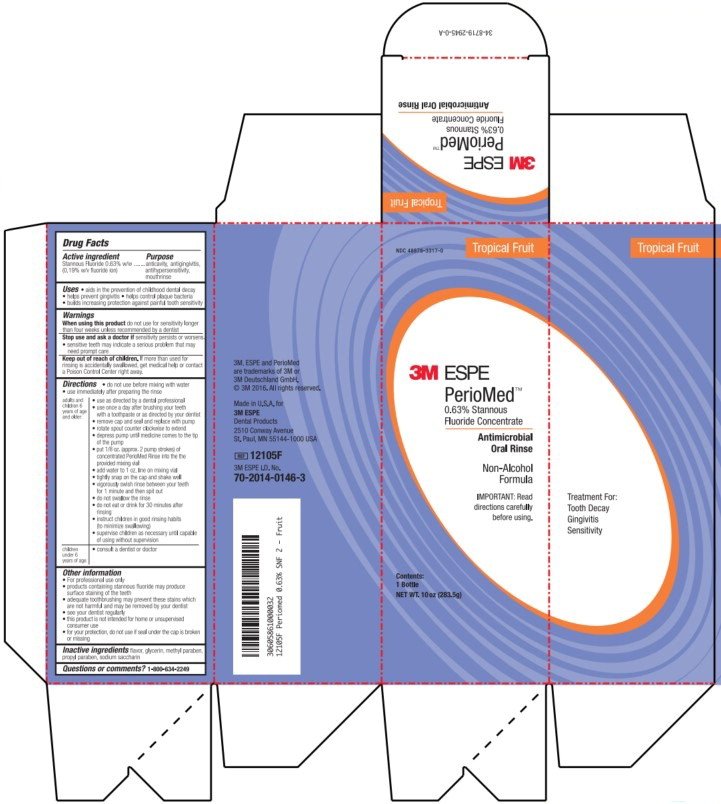
NDC 48878-3315-2
Mint
3M ESPE
PerioMed™
0.63% Stannous
Fluoride Concentrate
Antimicrobial
Oral Rinse
Non-Alcohol
Formula
IMPORTANT: Read
directions carefully
before using.
Contents:
1 Bottle
NET WT. 2.75 oz (78g)
| PERIOMED
stannous fluoride rinse |
|||||||||||||||||||
|
|||||||||||||||||||
|
|||||||||||||||||||
|
|||||||||||||||||||
|
|||||||||||||||||||
|
|||||||||||||||||||
|
|||||||||||||||||||
| PERIOMED
stannous fluoride rinse |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Labeler - 3M ESPE Dental Products (801390852) |
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.