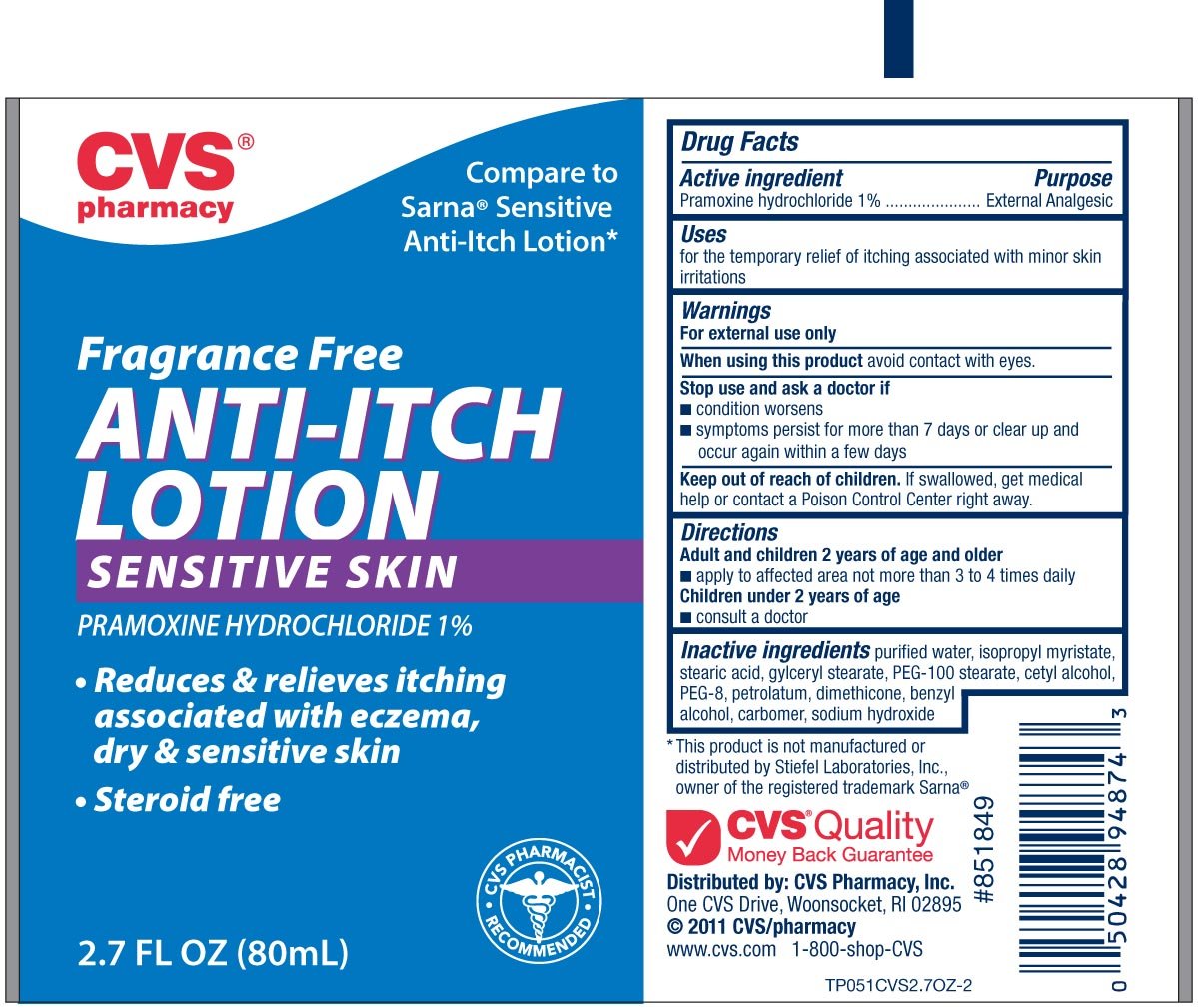
CVS Fragrance Free Anti-Itch
Dosage form: lotion
Ingredients: PRAMOXINE HYDROCHLORIDE 10mg in 1mL
Labeler: CVS Pharmacy
NDC code: 59779-052
Medically reviewed by Drugs.com. Last updated on Jun 28, 2023.
Active ingredient Purpose
Pramoxine Hydrochloride...................................External Analgesic
Pramoxine Hydrochloride...................................External Analgesic
Uses
For the temporary relief of itching associated with minor skin irritations
Keep out of reach of children. If swallowed, get medical help or contact a
Poison Control Center right away.
Uses
For the temporary relief of itching associated with minor skin irritations
Warnings
For external use only
When using this product
- avoid contact with eyes
Stop use and ask a doctor if
- condition worsens
- symptoms persist for more than 7 days or clear up and occur again within a few days.
Keep out of reach of children.If swallowed, get medical help or contact a
Poison Control Center right away.
For external use only
When using this product
- avoid contact with eyes
Stop use and ask a doctor if
- condition worsens
- symptoms persist for more than 7 days or clear up and occur again within a few days.
Keep out of reach of children.If swallowed, get medical help or contact a
Poison Control Center right away.
Directions
adults and children 2 years and older
- apply to affected area not more than 3 to 4 times daily
children under 2 years of age
- consult a doctor
Inactive Ingredients
water, isopropyl myristate, stearic acid, glyceryl stearate, PEG-100 stearate, cetyl alcohol,
PEG-8, petrolatum, dimethicone, benzyl alcohol, carbomer, sodium hydroxide
water, isopropyl myristate, stearic acid, glyceryl stearate, PEG-100 stearate, cetyl alcohol,
PEG-8, petrolatum, dimethicone, benzyl alcohol, carbomer, sodium hydroxide
| CVS FRAGRANCE FREE ANTI-ITCH
pramoxine hydrochloride lotion |
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
| Labeler - CVS Pharmacy (062312574) |
| Registrant - Pharma Pac, LLC (140807475) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Pharma Pac, LLC | 140807475 | manufacture | |
Document Id: c190935b-2f94-4c11-b531-f5726ea0904e
Set id: 34f7a899-2d36-4ad5-9cab-b697ffafda1c
Version: 1
Effective Time: 20100708
CVS Pharmacy
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.