The originating document has been archived. We cannot confirm the completeness, accuracy, or currency of the content.
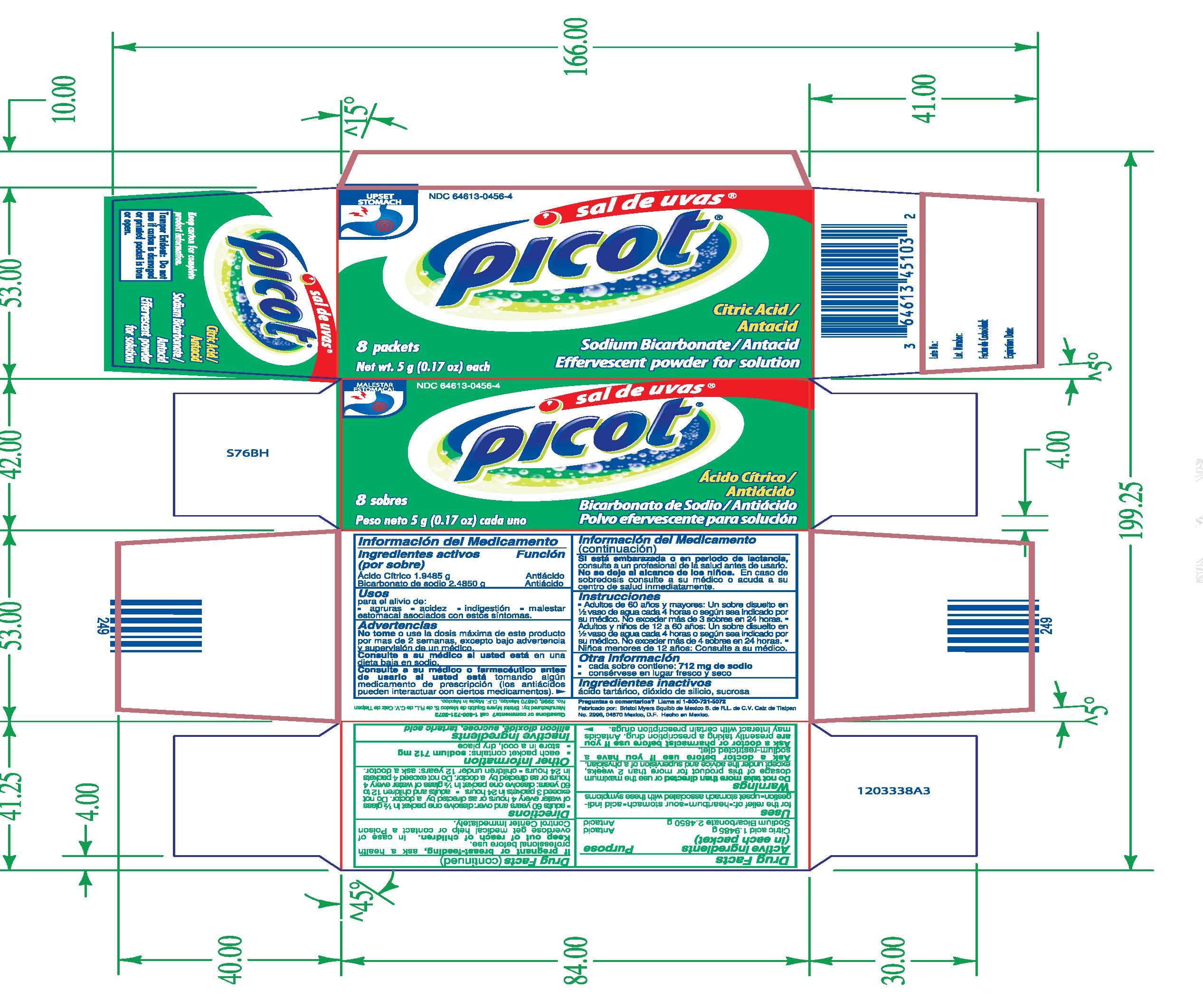
Sal de Uvas Picot
Dosage form: powder, for solution
Ingredients: CITRIC ACID MONOHYDRATE 1.9485g, SODIUM BICARBONATE 2.485g
Labeler: Bristol-Myers Squibb de Mexico, S. de R.L. de C.V.
NDC code: 64613-0456
Antacid
Active Ingredients
(in each packet)
Citric acid 1.9485 g
Sodium Bicarbonate 2.4850 g
Purpose
Antacid
Uses
for the relief of:
- Heartburn
- Sour stomach
- acid indigestion
- upset stomach associated with these symptoms
Warnings
Do not take more than directed or use the maximum dosage of this product for more than 2 weeks, except under the advice and supervision of a physician.
Ask a doctor before use if you have a sodium-restricted diet.
Ask a doctor or pharmacist before use if you are presently taking a prescription drug. Antacids may interact with certain prescription drugs.
If pregnant or breast-feeding, ask a health professional before use.
KEEP OUT OF REACH OF CHILDREN
In case of overdose get medical help or contact a Poison Control Center immediately.
Directions
- adults 60 years and over: dissolve one packet in 1/2 glass of water every 4 hours or as directed by a doctor. Do not exceed 3 packets in 24 hours
- adults and children 12 to 60 years: dissolve one packet in 1/2 glass of water every 4 hours or as directed by a doctor. Do not exceed 4 packets in 24 hours.
- children under 12 years: ask a doctor.
Other Information
- each packet contains: sodium 712 mg
- store in a cool, dry place
Inactive Ingredients
silicon dioxide, sucrose, tartaric acid
sal de uvas picot
Citric Acid/Antacid
Sodium Bicarbonate/Antacid
Effervescent powder for solution
8 packets
Net wt. 5 g (0.17 oz) each
Keep carton for complete product information.
Tamper Evident: Do not use if carton is damaged or printed packet is torn or open.
Questions or Comments? call 1-800-721-5072
Manufactured by: Bristol Myers Squibb de Mexico S. de R.L. de C.V. calz de Tialpan No. 2996, 04870 Mexico
Made in Mexico
Lot No.:
Expiration Date:
8 packets_1203338A3 9 jul 2015.jpg
| SAL DE UVAS PICOT
citrict acid, sodium bicarbonate powder, for solution |
|||||||||||||
|
|||||||||||||
|
|||||||||||||
|
|||||||||||||
|
|||||||||||||
|
|||||||||||||
| Labeler - Bristol-Myers Squibb de Mexico, S. de R.L. de C.V. (823646211) |
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.