The originating document has been archived. We cannot confirm the completeness, accuracy, or currency of the content.
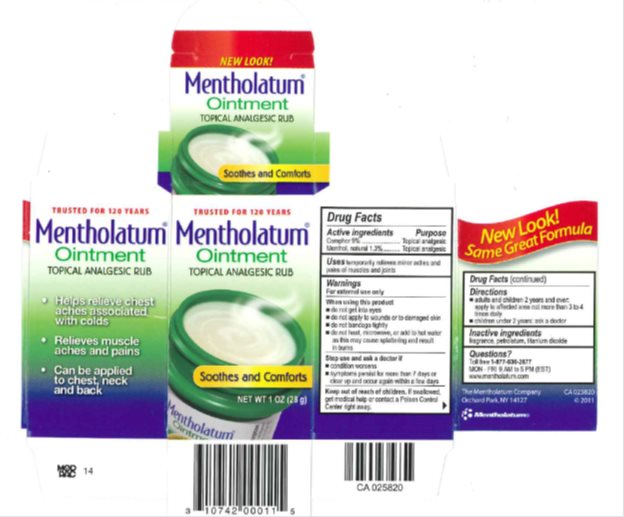
Mentholatum
Dosage form: ointment
Ingredients: CAMPHOR (SYNTHETIC) 90mg in 1g, MENTHOL, UNSPECIFIED FORM 13mg in 1g
Labeler: The Mentholatum Company
NDC code: 10742-0001
Camphor 9%
Menthol, natural 1.3%
Camphor - Topical Analgesic
Menthol, natural - Topical Analgesic
temporarily relieves minor aches and pains of muscles and joints
For external use only
- •
- do not heat
- •
- do not microwave
- •
- do not add to hot water or any container where heating water. May cause splattering and result in burns.
- •
- do not get into eyes
- •
- do not apply to wounds or to damaged skin
- •
- do not bandage tightly
- •
- condition worsens
- •
- symptoms persist for more than 7 days or clear up and occur again within a few days
If swallowed, get medical help or contact a Poison Control Center right away.
- •
- adults and children 2 years and over: apply to affected area not more than 3 to 4 times daily
- •
- children under 2 years: ask a doctor
fragrance, petrolatum, titanium dioxide
Toll free 1-877-636-2677 MON-FRI 9AM to 5PM (EST)
www.mentholatum.com
| MENTHOLATUM
camphor, menthol ointment |
|||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||
| Labeler - The Mentholatum Company (002105757) |
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.