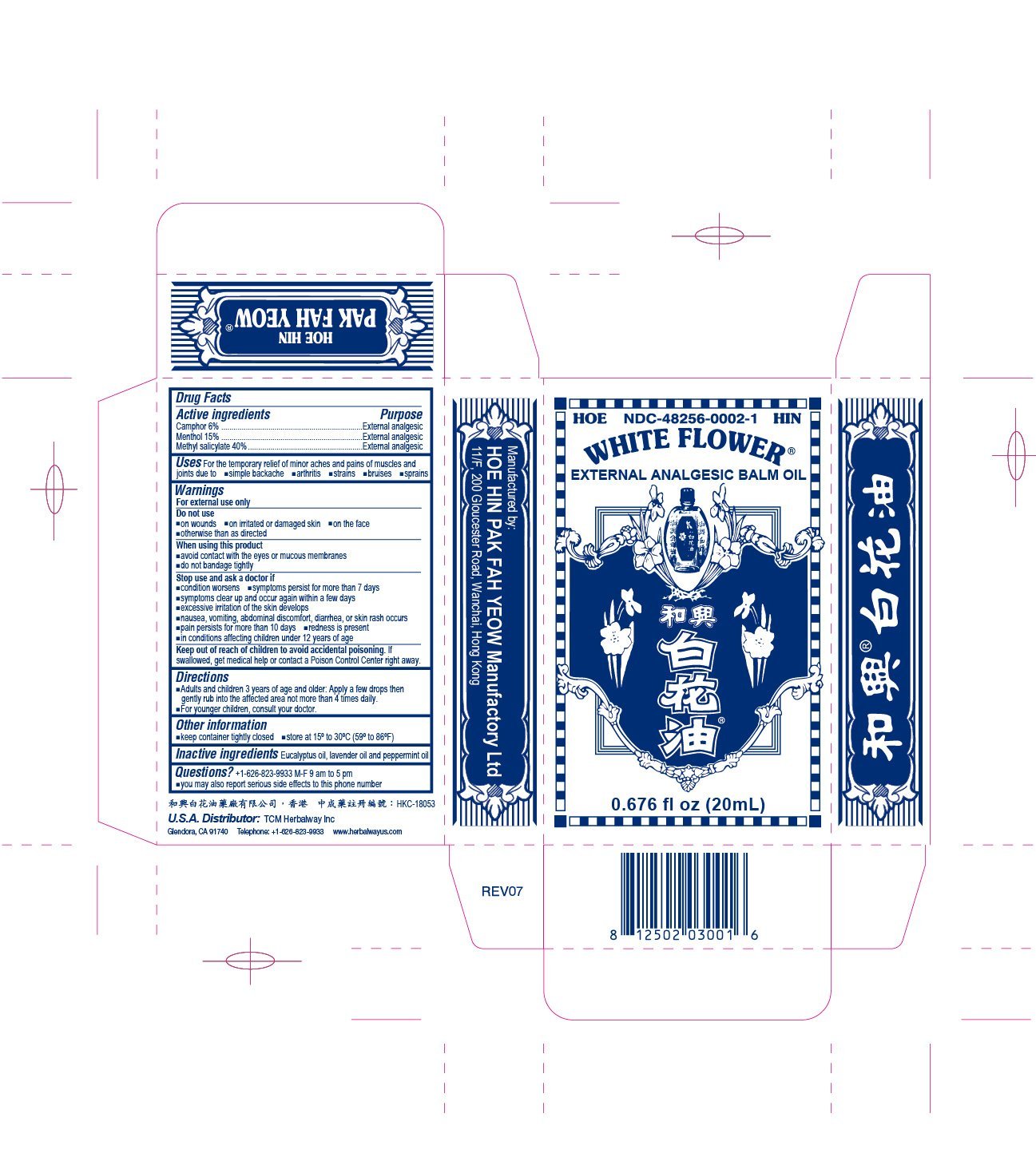
WHITE FLOWER ANALGESIC BALM
Dosage form: oil
Ingredients: CAMPHOR (SYNTHETIC) 6g in 100mL, MENTHOL 14.5g in 100mL, METHYL SALICYLATE 40.02g in 100mL
Labeler: HOE HIN PAK FAH YEOW MANUFACTORY LTD
NDC code: 48256-0002
Medically reviewed by Drugs.com. Last updated on Jul 3, 2023.
Active ingredients
Camphor 6%
Menthol 15%
Methyl salicylate 40%
Purpose
External analgesic
Do not use
- on wounds
- on irritated or damaged skin
- on the face
- otherwise than as directed
When using this product
- avoid contact with the eyes or mucous membranes
- do not bandage tightly
Stop use and ask a doctor if
- condition worsens
- symptoms persist for more than 7 days
- symptoms clear up and occur again within a few days
- excessive irritation of the skin develops
- nausea, vomiting, abdominal discomfort, diarrhea, or skin rash occurs
- pain persists for more than 10 days
- redness is present
- in conditions affecting children under 12 years of age
Keep out of reach of children to avoid accidental poisoning
If swallowed, get medical help or contact a Poison Control Center right away.
Directions
- Adults and children 3 years of age and older: Apply a few drops then gently rub into the affected area not more than 4 times daily.
- For younger children, consult your doctor.
Other information
- keep container tightly closed
- store at 15º to 30ºC (59º to 86ºF)
Inactive ingredients
Eucalyptus oil, lavender oil and peppermint oil.
Questions? +1-626-823-9933 M-F 9am to 5pm
- you may also report serious side effects to this phone number
Uses For the temporary relief of minor aches and pains of muscles and joints due to
- simple backache
- arthritis
- strains
- bruises
- sprains
Warnings
For external use only
| WHITE FLOWER ANALGESIC BALM
camphor, menthol and methyl salicylate oil |
|||||||||||||||||||
|
|||||||||||||||||||
|
|||||||||||||||||||
|
|||||||||||||||||||
|
|||||||||||||||||||
|
|||||||||||||||||||
| Labeler - HOE HIN PAK FAH YEOW MANUFACTORY LTD (686359001) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| MACAU-UNION PHARMACEUTICAL LTD | 448605614 | repack(48256-0002) | |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| HOE HIN PAK FAH YEOW MFY LTD | 686212961 | manufacture(48256-0002) | |
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.