FDA Approves Onzetra Xsail
FDA Approves Onzetra Xsail (sumatriptan nasal powder) for the Acute Treatment of Migraine
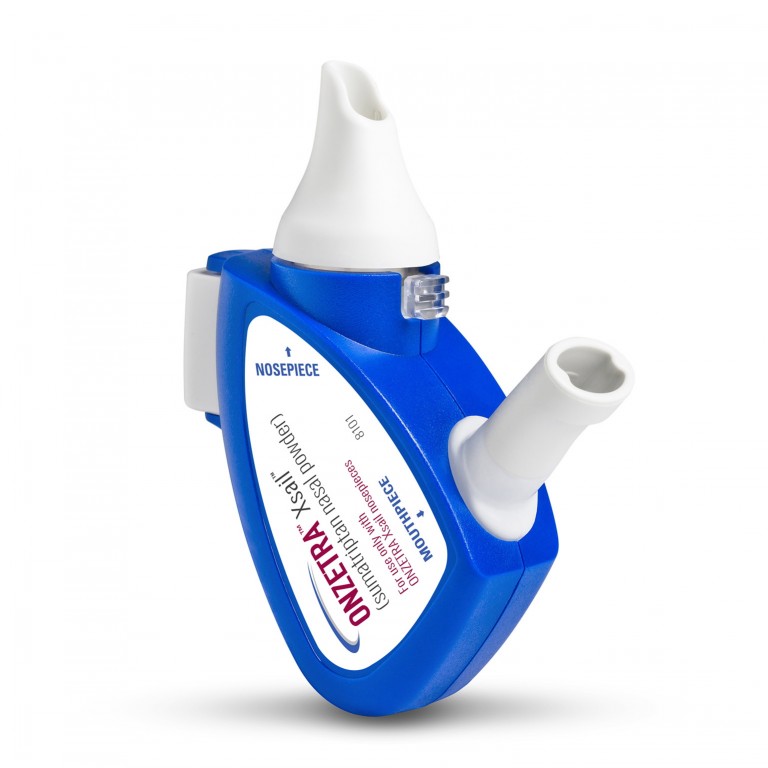
ALISO VIEJO, Calif., January 28, 2016 -- Avanir Pharmaceuticals, Inc. today announced that the U.S. Food and Drug Administration (FDA) has approved Onzetra Xsail (sumatriptan nasal powder), formerly known as AVP-825, for the acute treatment of migraine with or without aura in adults. Onzetra Xsail is an intranasal medication delivery system consisting of a low-dose (22mg) of sumatriptan powder that is delivered utilizing the novel Xsail™ Breath Powered Delivery Device. Onzetra Xsail is a fast-acting dry powder formulation of sumatriptan, the most commonly prescribed migraine medication.
“While there are many acute migraine treatment options available, more than 70% of patients are not fully satisfied with their current migraine treatment. Given this high dissatisfaction, there remains an unmet need to provide patients with fast-acting, well tolerated therapies that deliver consistent relief,” said Stewart Tepper, M.D., professor of neurology at the Geisel School of Medicine at Dartmouth.
“Onzetra Xsail provides a new and much needed treatment option for what can be a debilitating condition for millions of people,” said Roger K. Cady, M.D., director of the Headache Care Center and associate executive chairman of the National Headache Foundation. “The Xsail Breath Powered Delivery Device allows the medication to be deposited deep into the nose, an area that is rich with blood vessels. By delivering the medication here, Onzetra Xsail provides targeted and efficient delivery with the potential for fast, consistent relief, while also limiting the amount of medicine that goes down the back of the throat.”
“We are excited about this major milestone for Avanir as the approval of Onzetra Xsail represents our second approved product. We expect to make Onzetra Xsail available to patients in the coming months,” said Rohan Palekar, president and CEO of Avanir. “We continue to be focused on building a leading CNS biopharmaceutical company and the addition of Onzetra Xsail to our product portfolio takes us closer to achieving that goal.”
About the Onzetra Xsail Clinical Trial Program
The approval of Onzetra Xsail is based on data from phase II and phase III trials, safety data from more than 300 patients, and reference data from the extensive clinical use of sumatriptan over the past 20 years. In the pivotal TARGET trial, 230 migraine sufferers were randomized to self-administer either Onzetra or placebo using the Xsail Breath Powered Delivery Device when they had moderate to severe migraine pain. Pain scores were then assessed at various time points after administration. Pain was evaluated using a four point scale with headache relief defined as a reduction from moderate (grade 2) or severe (grade 3) pain to mild (grade 1) or complete relief (grade 0). Study results demonstrated that a significantly greater proportion of Onzetra Xsail patients reported headache relief at 30 minutes (41.7% vs. 26.9%, P=0.03) and at every time point up to two hours post-dose compared with those using the placebo device (67.6% vs. 45.2%, P=0.002). The treatment was well tolerated with a low incidence of adverse events (AEs). In addition, in pooled studies, the treatment was well tolerated with a low incidence of AEs, with the most common AEs being abnormal product taste (20%), nasal discomfort (11%), rhinorrhea (5%) and rhinitis (2%); local AEs were transient and almost exclusively mild to moderate in severity.
About Onzetra Xsail
Onzetra Xsail is an intranasal medication delivery system consisting of low-dose (22mg) sumatriptan powder, the most commonly prescribed migraine medication. The medication is delivered intranasally utilizing the novel Xsail Breath Powered Delivery Device. It is a fast-acting dry-powder intranasal form of sumatriptan for the acute treatment of migraine. Sumatriptan is the most widely used prescription migraine medication and has been used safely for over 20 years. Sumatriptan is contraindicated for certain patients, including those with a history of coronary artery disease (CAD) or coronary vasospasm.
The breath powered delivery technology is activated by a user's breath to propel medication deep into the nasal cavity where absorption is more efficient and consistent. The user exhales into the device, automatically closing the soft palate and sealing off the nasal cavity. Through a sealing nosepiece placed into the nostril, the exhaled breath carries medication from the device directly into one side of the nose. Narrow nasal passages are gently expanded and medication is dispersed deep into the nasal cavity reaching areas where it can be rapidly absorbed. As the medication is delivered, the air flows around to the opposite side of the nasal cavity and exits through the other nostril. Closure of the soft palate helps prevent swallowing and reduce GI absorption.
Important Safety Information
Onzetra Xsail is indicated for the acute treatment of migraine with or without aura in adults.
Limitations of Use
- Use only if a clear diagnosis of migraine has been established. If a patient has no response to the first migraine attack treated with Onzetra Xsail, reconsider the diagnosis of migraine before treatment of subsequent attacks with Onzetra Xsail.
- Onzetra Xsail is not indicated for the prevention of migraine attacks.
- Safety and effectiveness of Onzetra Xsail have not been established for the treatment of cluster headache.
Onzetra is contraindicated in patients with:
- Ischemic coronary artery disease (CAD) or coronary artery vasospasm, including Prinzmetal’s angina; or Wolff-Parkinson-White syndrome or arrhythmias associated with other cardiac accessory conduction pathway disorders
- History of stroke, transient ischemic attack (TIA), or history of hemiplegic or basilar migraine; peripheral vascular disease; ischemic bowel disease; or uncontrolled hypertension
- Recent (i.e., within 24 hours) use of ergotamine-containing or ergot-type medication, or another 5-HT1 agonist; or concurrent or recent (within 2 weeks) use of a MAO-A inhibitor
- Known hypersensitivity to sumatriptan (angioedema and anaphylaxis seen) or severe hepatic impairment
Other serious adverse events associated with the use of sumatriptan or 5-HT1 agonists include: myocardial ischemia/infarction, Prinzmetal's angina, arrhythmias; chest, throat, neck and/or jaw pain/tightness/pressure; cerebral hemorrhage, subarachnoid hemorrhage, and stroke; peripheral vascular ischemia, gastrointestinal vascular ischemia/infarction, and Raynaud’s syndrome, medication overuse headache; serotonin syndrome; significant elevation in blood pressure; anaphylactic/anaphylactoid reactions; and seizures.
In clinical trials, the most common adverse reactions (≥ 2% and > placebo) were abnormal taste, nasal discomfort, rhinorrhea, and rhinitis
Advise patients to carefully read the Patient Information and Instructions for Use prior to using Onzetra.
For additional important safety information about Onzetra, please see full Prescribing Information.
About Migraine
Migraine represents an area of significant unmet medical need. According to the Centers for Disease Control and Prevention, over 37 million Americans suffer from migraine headaches. The triptan class of medications is generally considered the standard of care with over 14 million prescriptions written annually. Sumatriptan is the class leader with a market share of over 50% making it the most commonly prescribed migraine drug in the United States. 100 mg tablets are the most commonly prescribed form of sumatriptan. In a published survey of 688 migraine sufferers, 71% were found to be not fully satisfied with their migraine treatment. [i] As a result, many migraine sufferers are seeking new, fast-acting, well tolerated treatment options.
About Avanir Pharmaceuticals, Inc.
Avanir Pharmaceuticals, Inc. is a biopharmaceutical company focused on bringing innovative medicines to patients with central nervous system disorders of high unmet medical need. As part of our commitment, we have extensively invested in our pipeline and are dedicated to advancing medicines that can substantially improve the lives of patients and their loved ones. For more information about Avanir, please visit http://www.avanir.com.Avanir is a subsidiary of Otsuka America, Inc. (OAI), a holding company established in the U.S. in 1989. OAI is wholly owned by Otsuka Pharmaceutical Co., Ltd., a global healthcare company with the corporate philosophy: 'Otsuka-people creating new products for better health worldwide.'
Otsuka Pharmaceutical is a leading firm in the challenging area of mental health and also has products and research programs for several under-addressed diseases including tuberculosis, a significant global public health issue. These commitments illustrate more powerfully than words how Otsuka is a "big venture" company at heart, applying a youthful spirit of creativity in everything it does.
Otsuka Pharmaceutical and its affiliates employ approximately 30,000 people globally, and the company welcomes you to visit its global website at: http://www.otsuka.co.jp/en/index.php
Avanir® and Onzetra Xsail are trademarks or registered trademarks of Avanir Pharmaceuticals, Inc. in the United States and other countries.
SOURCE Avanir Pharmaceuticals, Inc.
Posted: January 2016
Related articles
- Avanir Pharmaceuticals Receives Complete Response Letter (CRL) from FDA on AVP-825 NDA - November 26, 2014
- Avanir Pharmaceuticals Announces Preliminary Feedback from the FDA on AVP-825 for the Acute Treatment of Migraine - November 7, 2014
- Avanir Pharmaceuticals Announces Publication of Pivotal Phase III Results from AVP-825 Acute Migraine Study - October 30, 2014
- Avanir Pharmaceuticals Announces Acceptance of NDA for AVP-825 for the Acute Treatment of Migraine - March 26, 2014
- Avanir Pharmaceuticals Announces Submission of New Drug Application for AVP-825 for the Acute Treatment of Migraine - January 30, 2014
Onzetra Xsail (sumatriptan) FDA Approval History
More news resources
- FDA Medwatch Drug Alerts
- Daily MedNews
- News for Health Professionals
- New Drug Approvals
- New Drug Applications
- Drug Shortages
- Clinical Trial Results
- Generic Drug Approvals
Subscribe to our newsletter
Whatever your topic of interest, subscribe to our newsletters to get the best of Drugs.com in your inbox.
