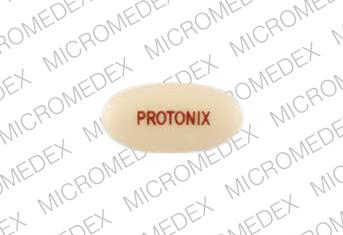
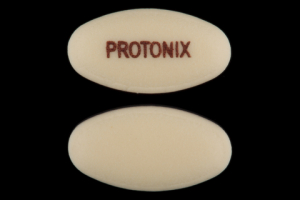
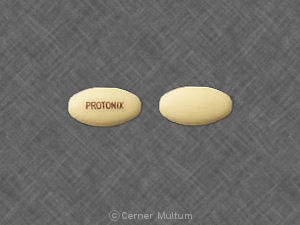
PROTONIX Pill - yellow oval, 12mm
Generic Name: pantoprazole
Pill with imprint PROTONIX is Yellow, Oval and has been identified as Protonix 40 mg. It is supplied by Wyeth.
Protonix is used in the treatment of Barrett's Esophagus; Erosive Esophagitis; Duodenal Ulcer; GERD; Helicobacter Pylori Infection and belongs to the drug class proton pump inhibitors. There is no proven risk in humans during pregnancy. Protonix 40 mg is not a controlled substance under the Controlled Substances Act (CSA).
Images for PROTONIX
Protonix
- Generic Name
- pantoprazole
- Imprint
- PROTONIX
- Strength
- 40 mg
- Color
- Yellow
- Size
- 12.00 mm
- Shape
- Oval
- Availability
- Prescription only
- Drug Class
- Proton pump inhibitors
- Pregnancy Category
- B - No proven risk in humans
- CSA Schedule
- Not a controlled drug
- Labeler / Supplier
- Wyeth
- Inactive Ingredients
-
calcium stearate,
mannitol,
ferric oxide yellow,
methacrylic acid - ethyl acrylate copolymer (1:1) type a,
polysorbate 80,
povidone k25,
povidone k90,
propylene glycol,
sodium carbonate,
sodium lauryl sulfate,
titanium dioxide,
triethyl citrate,
hypromellose 2910 (6 mPa.s)
Note: Inactive ingredients may vary.
Labelers / Repackagers
| NDC Code | Labeler / Repackager |
|---|---|
| 00008-0841 | Wyeth |
| 00008-0607 (Discontinued) | Wyeth |
| 54569-5118 (Discontinued) | A-S Medication Solutions, LLC (repackager) |
| 54868-4271 (Discontinued) | Physicians Total Care Inc. (repackager) |
| 66116-0241 | Medvantx Inc. (repackager) |
| 52959-0819 | H.J. Harkins Company, Inc. (repackager) |
| 49999-0756 (Discontinued) | Lake Erie Medical and Surgical Supply (repackager) |
| 67544-0402 | Prepak Systems Inc. (repackager) |
| 49999-0481 | Lake Erie Medical and Surgical Supply (repackager) |
| 66267-0479 | Nucare Pharmaceuticals Inc. (repackager) |
More about Protonix (pantoprazole)
- Check interactions
- Compare alternatives
- Pricing & coupons
- Reviews (82)
- Drug images
- Side effects
- Dosage information
- Patient tips
- During pregnancy
- Generic availability
- Support group
- Drug class: proton pump inhibitors
- Breastfeeding
- En español
Patient resources
- Protonix drug information
- Protonix (Pantoprazole Intravenous) (Advanced Reading)
- Protonix (Pantoprazole Oral) (Advanced Reading)
- Protonix (Pantoprazole Delayed-Release Granules)
- Protonix (Pantoprazole Delayed-Release Tablets)
Professional resources
Other formulations
Related treatment guides
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.
