DermaRite Industries Issues Recall of DermaKleen, Dermasarra, Kleenfoam, and Perigiene Products Due to Burkholderia cepacia Contamination
Audience: Consumer
August 8, 2025 -- DermaRite Industries, LLC is voluntarily recalling individual lots of products in the table below due to microbial contamination identified as Burkholderia cepecia.
Risk Statement: Burkholderia Cepacia Complex in these products may result in serious and life-threatening infections. The contaminated products may be used by immunosuppressed individuals or by people attending to immunosuppressed individuals. In healthy individuals with minor skin lesions the use of the product will more likely result in local infections, whereas in immunocompromised individuals the infection is more likely to spread into blood stream leading to life-threatening sepsis. To date, DermaRite has not received any reports of adverse events related to this recall.
DermaKleen is an OTC Healthcare antiseptic lotion soap with Vitamin E indicated for handwashing to decrease bacteria on the skin.
DermaSarra is an OTC External analgesic indicated for temporary relief of itching associated with minor skin irritations due to: dry skin, insect bites, detergents, sunburn.
KleenFoam is an OTC Antimicrobial foam soap with Aloe Vera indicated for handwashing to decrease bacteria on the skin after changing diapers, after assisting ill people, or before contact with a person under medical care or treatment.
PeriGiene is an OTC Antiseptic cleanser indicated for use in the perineal area.
The recalled products were distributed nationwide in the United States and in Puerto Rico.
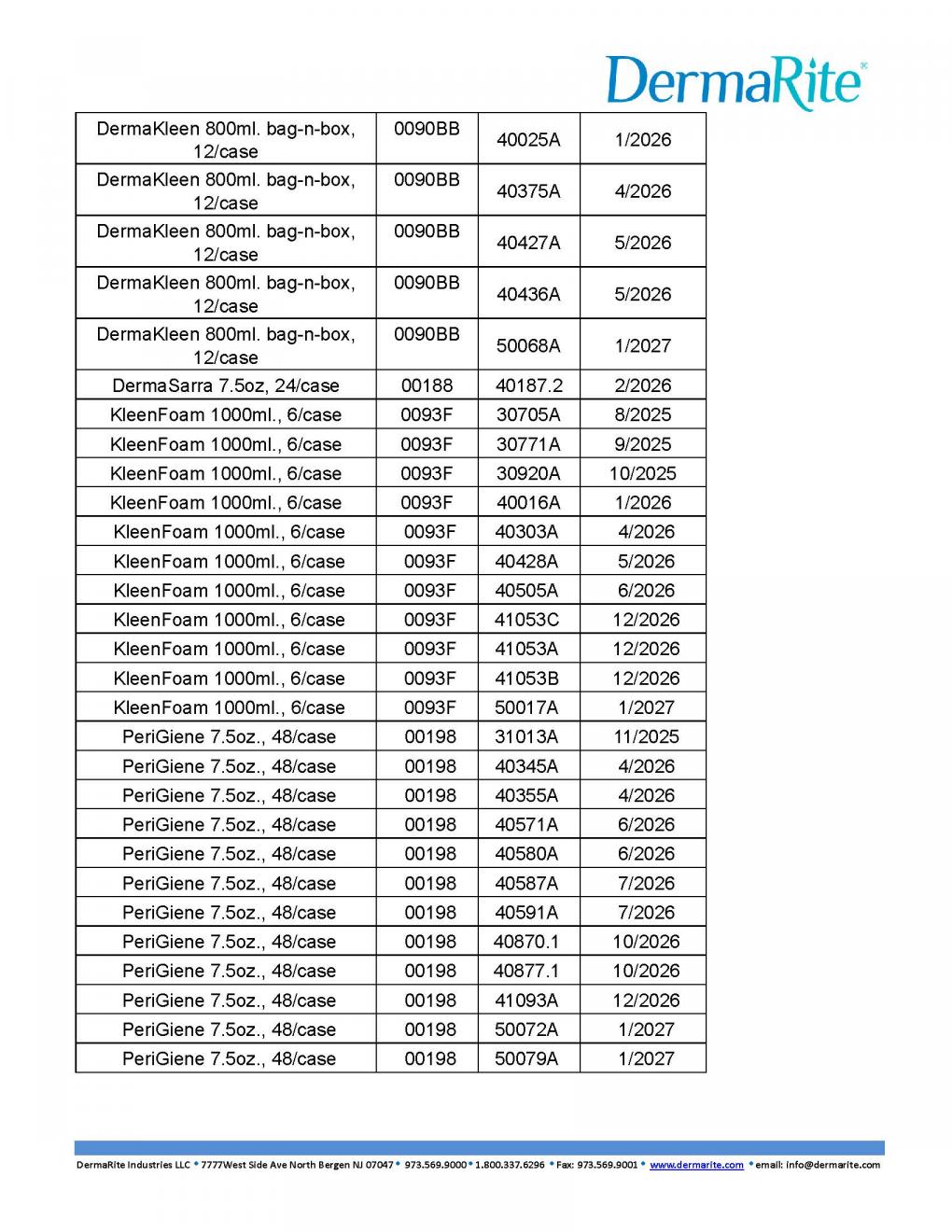
Recalled Product Information: (see Table image below)
DermaRite has notified its distributors and customers by e-mail to immediately examine available inventory and destroy all affected products in accordance with each facility’s process.
Consumers with questions regarding this recall can call Mary Goldberg at 973-569-9000 x104 Monday through Friday, 9:00 am – 5:00 pm EST or email voluntary.action@dermarite.com.
Consumers should contact their physician or healthcare provider if they have experienced any problems that may be related to taking or using this product.
Adverse reactions or quality problems experienced with the use of this product may be reported to the FDA’s MedWatch Adverse Event Reporting program either online, by regular mail, or by fax.
- Complete and submit the report online: www.fda.gov/medwatch/report.htm
- Regular Mail or Fax: Download form www.fda.gov/MedWatch/getforms.htm or call 1-800-332-1088 to request a reporting form, then complete and return to the address on the pre-addressed form or submit by fax to 1-800-FDA-0178.
This recall is being conducted with the knowledge of the U.S. Food and Drug Administration.






More news resources
- FDA Medwatch Drug Alerts
- Daily MedNews
- News for Health Professionals
- New Drug Approvals
- New Drug Applications
- Drug Shortages
- Clinical Trial Results
- Generic Drug Approvals
Subscribe to our newsletter
Whatever your topic of interest, subscribe to our newsletters to get the best of Drugs.com in your inbox.
