Imlygic Dosage
Generic name: TALIMOGENE LAHERPAREPVEC 1000000[PFU] in 1mL
Dosage form: injection, suspension
Drug class: Miscellaneous antineoplastics
Medically reviewed by Drugs.com. Last updated on Feb 10, 2023.
For intralesional injection only. Do not administer intravenously.
Dose
Administer IMLYGIC by injection into cutaneous, subcutaneous, and/or nodal lesions that are visible, palpable, or detectable by ultrasound guidance.
IMLYGIC is provided in single-use vials of 1 mL each in two different dose strengths:
- 106 (1 million) plaque-forming units (PFU) per mL (light green cap) – for initial dose only
- 108 (100 million) PFU per mL (royal blue cap) – for all subsequent doses
Recommended Dose and Schedule
The total injection volume for each treatment visit should not exceed 4 mL for all injected lesions combined. It may not be possible to inject all lesions at each treatment visit or over the full course of treatment. Previously injected and/or uninjected lesion(s) may be injected at subsequent treatment visits. The initial recommended dose is up to 4 mL of IMLYGIC at a concentration of 106 (1 million) PFU per mL. The recommended dose for subsequent administrations is up to 4 mL of IMLYGIC at a concentration of 108 (100 million) PFU per mL. The recommended dosing schedule for IMLYGIC is shown in Table 1.
| Treatment | Treatment Interval | Maximum Injection Volume per Treatment Visit (all lesions combined) |
Dose Strength | Prioritization of Lesions to be Injected |
| Initial | – | 4 mL | 106 (1 million) PFU per mL |
|
| Second | 3 weeks after initial treatment | 4 mL | 108 (100 million) PFU per mL |
|
| All subsequent treatments (including reinitiation) | 2 weeks after previous treatment | 4 mL | 108 (100 million) PFU per mL |
|
Dose Volume Determination (per Lesion)
Use Table 2 to determine the volume of IMLYGIC injection for each lesion.
| Lesion Size (longest dimension) |
Injection Volume |
| > 5 cm | up to 4 mL |
| > 2.5 cm to 5 cm | up to 2 mL |
| > 1.5 cm to 2.5 cm | up to 1 mL |
| > 0.5 cm to 1.5 cm | up to 0.5 mL |
| ≤ 0.5 cm | up to 0.1 mL |
When lesions are clustered together, inject them as a single lesion according to Table 2.
Continue IMLYGIC treatment for at least 6 months unless other treatment is required or until there are no injectable lesions to treat.
Reinitiate IMLYGIC treatment if new unresectable cutaneous, subcutaneous, or nodal lesions appear after a complete response.
Preparation and Handling
Healthcare providers who are immunocompromised or pregnant should not prepare or administer IMLYGIC and should not come into direct contact with the IMLYGIC injection sites, dressings, or body fluids of treated patients.
Avoid accidental exposure to IMLYGIC and follow below instructions for preparation, administration, and handling of IMLYGIC:
- Wear personal protective equipment (protective gown or laboratory coat, safety glasses or face shield, and gloves) while preparing or administering IMLYGIC.
- Avoid accidental exposure to IMLYGIC, especially contact with skin, eyes, and mucous membranes.
○ Cover any exposed wounds before handling.
○ In the event of an accidental occupational exposure (e.g., through a splash to the eyes or mucous membranes), flush with clean water for at least 15 minutes.
○ In the event of exposure to broken skin or needle stick, clean the affected area thoroughly with soap and water and/or a disinfectant. - Clean all surfaces that may have come in contact with IMLYGIC and treat all IMLYGIC spills with a virucidal agent such as 1% sodium hypochlorite or 70% isopropyl alcohol and blot using absorbent materials.
- Dispose of all materials that may have come in contact with IMLYGIC (e.g., vial, syringe, needle, cotton gauze, gloves, masks, or dressings) as biohazardous waste.
- Advise patients to place used dressings and cleaning materials into a sealed plastic bag and dispose in household waste.
Thawing IMLYGIC Vials
- 1.
- Determine the total volume required for injection, up to 4 mL.
- 2.
- Thaw frozen IMLYGIC vials at room temperature [20°C to 25°C (68°F to 77°F)] until IMLYGIC is liquid. The time to achieve complete vial thaw is expected to be 30 to 70 minutes, depending on the ambient temperature. Do not expose the vial to higher temperatures. Keep the vial in original carton during thawing.
- 3.
- Swirl gently. Do NOT shake.
- 4.
- After thawing, administer IMLYGIC immediately or store in its original vial and carton, as follows:
- Thawed IMLYGIC is stable when stored at temperatures of 2°C (36°F) up to 25°C (77°F) protected from light in its original vial and carton for the storage times specified in Table 3. Do not exceed the storage times specified in Table 3.
- IMLYGIC must not be refrozen once it is thawed. Discard any thawed IMLYGIC in the vial stored longer than the specified times in Table 3.
| 106 (1 million) PFU/mL | 108 (100 million) PFU/mL | |
| 2°C to 8°C (36°F to 46°F) | 24 hours | 1 week (7 days) |
| up to 25°C (77°F) | 12 hours | 24 hours |
- 5.
- Prepare sterile syringes and needles. A detachable 18-26 G needle or nondetachable 22-26 G staked needle (which minimizes hold up volume) may be used for IMLYGIC withdrawal and a detachable or nondetachable staked needle of 22–26 G may be used for injection. Small unit syringes (e.g., 0.5 mL insulin syringes) are recommended for better injection control.
- 6.
- Using aseptic technique, remove the vial cap and withdraw the product from the vial into the syringe(s), noting the total volume. Avoid generating aerosols when loading syringes with product and use a biologic safety cabinet if available.
Administration
Follow the steps below to administer IMLYGIC to patients:
Pre-Injection
- Clean the lesion and surrounding areas with an alcohol swab and let dry.
- Treat the injection site with a topical or local anesthetic agent, if necessary. Do not inject anesthetic agent directly into the lesion. Inject anesthetic agent around the periphery of the lesion.
Injection
- Inject IMLYGIC intralesionally into cutaneous, subcutaneous, and/or nodal lesions that are visible, palpable, or detectable by ultrasound guidance. Using a single insertion point, inject IMLYGIC along multiple tracks as far as the radial reach of the needle allows within the lesion to achieve even and complete dispersion. Multiple insertion points may be used if a lesion is larger than the radial reach of the needle.
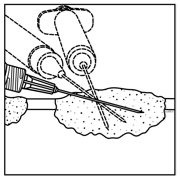
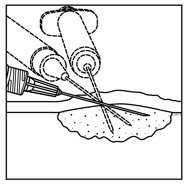
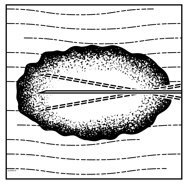
Figure 1: Injection administration for cutaneous lesions Figure 2: Injection administration for subcutaneous lesions Figure 3: Injection administration for nodal lesions - Inject IMLYGIC evenly and completely within the lesion by pulling the needle back without exiting the lesion. Redirect the needle as many times as necessary while injecting the remainder of the dose of IMLYGIC. Continue until the full dose is evenly and completely dispersed.
- When removing the needle, withdraw it from the lesion slowly to avoid leakage of IMLYGIC at the insertion point.
- Repeat steps 1-2 under pre-injection and steps 1-3 under injection for other lesions to be injected.
- Use a new needle any time the needle is completely removed from a lesion and each time a different lesion is injected.
Post-Injection
- Apply pressure to the injection site(s) with sterile gauze for at least 30 seconds.
- Swab the injection site(s) and surrounding area with alcohol.
- Change gloves and cover the injected lesion(s) with an absorbent pad and dry occlusive dressing.
- Wipe the exterior of occlusive dressing with alcohol.
- Advise patients to:
- Keep the injection site(s) covered for at least the first week after each treatment visit or longer if the injection site is weeping or oozing.
- Replace the dressing if it falls off.
More about Imlygic (talimogene laherparepvec)
- Check interactions
- Compare alternatives
- Pricing & coupons
- Side effects
- During pregnancy
- FDA approval history
- Drug class: miscellaneous antineoplastics
- En español
Patient resources
Professional resources
Related treatment guides
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.




