Erythrocin Injection USP Dosage
Generic name: ERYTHROMYCIN LACTOBIONATE 500mg in 100mL
Dosage form: injection, powder, lyophilized, for solution
Drug class: Macrolides
Medically reviewed by Drugs.com. Last updated on Nov 16, 2022.
For the treatment of severe infections in adults and pediatric patients, the recommended intravenous dose of erythromycin lactobionate is 15 to 20 mg/kg/day. Higher doses, up to 4 g/day, may be given for severe infections.
Administration of doses of ≥4 g/day may increase the risk for the development of erythromycin-induced hearing loss in elderly patients, particularly those with reduced renal or hepatic function. Erythrocin Lactobionate-IV (erythromycin lactobionate for injection, USP) in the ADD-Vantage system must be administered by intermittent intravenous infusion only. Due to the irritative properties of erythromycin, IV push is an unacceptable route of administration.
Intravenous erythromycin should be replaced by oral erythromycin as soon as possible.
The drug should be administered as a single dose from the ADD-Vantage flexible diluent container. Discard any unused portion.
For intermittent infusion: administer one-fourth the total daily dose of erythromycin lactobionate by intravenous infusion in 20 to 60 minutes at intervals not greater than every six hours. The final diluted solution of erythromycin lactobionate is prepared to give a concentration of 1 to 5 mg/mL. No less than 100 mL of IV diluent should be used. Infusion should be sufficiently slow to minimize pain along the vein.
For treatment of acute pelvic inflammatory disease caused by N. Gonorrhoeae, in female patients hypersensitive to penicillins, administer 500 mg erythromycin lactobionate every six hours for three days, followed by oral administration of 250 mg erythromycin stearate or base every six hours for seven days.
For treatment of Legionnaires' Disease: Although optimal doses have not been established, doses utilized in reported clinical data were 1 to 4 grams daily in divided doses.
Administration of doses of ≥4 g/day may increase the risk for the development of erythromycin-induced hearing loss in elderly patients, particularly those with reduced renal or hepatic function.
In the treatment of Group A beta-hemolytic streptococcal infections of the upper respiratory tract (e.g., tonsillitis or pharyngitis), the therapeutic dosage of erythromycin should be administered for ten days. The American Heart Association suggests a dosage of 250 mg of erythromycin orally, twice a day in long-term prophylaxis of streptococcal upper respiratory tract infections for the prevention of recurring attacks of rheumatic fever in patients allergic to penicillin and sulfonamides.1
In prophylaxis against bacterial endocarditis (See INDICATIONS AND USAGE) the oral regimen for penicillin allergic patients is erythromycin 1 gram, 1 hour before the procedure followed by 500 mg six hours later.2
Preparation of Solution:
The Erythrocin Lactobionate-IV ADD-Vantage vial may be used with either 0.9% Sodium Chloride Injection, USP, or 5% Dextrose Injection, USP in the ADD-Vantage flexible diluent container. The 500 mg ADD-Vantage vials must be used as single doses with the 100 mL ADD-Vantage flexible diluent containers. The resulting solution will contain erythromycin activity equal to approximately 5 mg/mL.
Do not administer unless solution is clear and container is undamaged. Discard any unused portion.
INSTRUCTIONS FOR USE
To Use Vial in ADD-Vantage Flexible Diluent Container
To Open:
Peel overwrap at corner and remove solution container. Some opacity of the plastic due to moisture absorption during the sterilization process may be observed. This is normal and does not affect the solution quality or safety. The opacity will diminish gradually.
To Assemble Vial and Flexible Diluent Container:
(Use Aseptic Technique)
- •
- Remove the protective covers from the top of the vial and the vial port on the diluent container as follows:
- a.
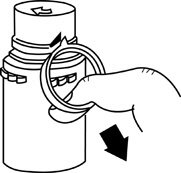
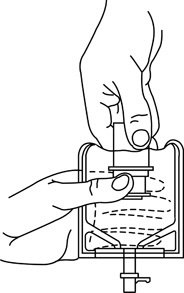
- To remove the breakaway vial cap, swing the pull ring over the top of the vial and pull down far enough to start the opening (See FIGURE 1.), then pull straight up to remove the cap. (See FIGURE 2.) NOTE: Do not access vial with syringe.
Figure 1 Figure 2
-
- o
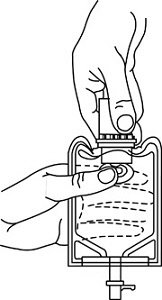
- To remove the vial port cover, grasp the tab on the pull ring, pull up to break the three tie strings, then pull back to remove the cover. (See FIGURE 3.)
- •
- Screw the vial into the vial port until it will go no further. THE VIAL MUST BE SCREWED IN TIGHTLY TO ASSURE A SEAL. This occurs approximately 1/2 turn (180°) after the first audible click. (See FIGURE 4.) The clicking sound does not assure a seal; the vial must be turned as far as it will go.
NOTE: Once vial is seated, do not attempt to remove. (See FIGURE 4.)
Figure 3 Figure 4
- •
- Recheck the vial to assure that it is tight by trying to turn it further in the direction of assembly.
- •
- Label appropriately.
To Reconstitute the Drug:
- •
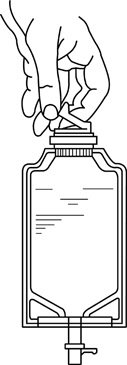
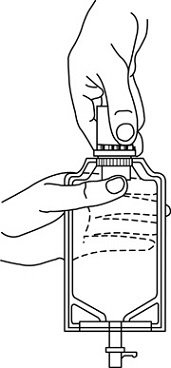
- Squeeze the bottom of the diluent container gently to inflate the portion of the container surrounding the end of the drug vial.
- •
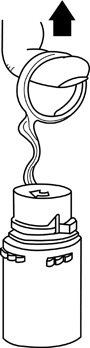
- With the other hand, push the drug vial down into the container telescoping the walls of the container. Grasp the inner cap of the vial through the walls of the container. (See FIGURE 5.)
- •
- Pull the inner cap from the drug vial. (See FIGURE 6.) Verify that the rubber stopper has been pulled out, allowing the drug and diluent to mix.
- •
- Mix container contents thoroughly and use within the specified time.
Figure 5 Figure 6
Preparation for Administration:
(Use Aseptic Technique)
- •
- Confirm the activation and admixture of vial contents.
- •
- Check for leaks by squeezing container firmly. If leaks are found, discard unit as sterility may be impaired.
- •
- Close flow control clamp of administration set.
- •
- Remove cover from outlet port at bottom of container.
- •
- Insert piercing pin of administration set into port with a twisting motion until the pin is firmly seated.
NOTE: See full directions on administration set carton.
- •
- Lift the free end of the hanger loop on the bottom of the vial, breaking the two tie strings. Bend the loop outward to lock it in the upright position, then suspend container from hanger.
- •
- Squeeze and release drip chamber to establish proper fluid level in chamber.
- •
- Open flow control clamp and clear air from set. Close clamp.
- •
- Attach set to venipuncture device. If device is not indwelling, prime and make venipuncture.
- •
- Regulate rate of administration with flow control clamp.
WARNING: Do not use flexible containers in series connections.
Stability:
In 0.9% Sodium Chloride Injection, USP
The final diluted solution of erythromycin lactobionate should be completely administered within 8 hours in order to assure proper potency.
In 5% Dextrose Injection, USP
The final diluted solution of erythromycin lactobionate should be completely administered within 2 hours in order to assure proper potency.
No drug or chemical agent should be added to an Erythrocin Lactobionate-IV fluid admixture unless its effect on the chemical and physical stability of the solution has first been determined.
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit.
WARNING: Do not use flexible container in series connections.
Frequently asked questions
- What causes black hairy tongue?
- What are enteric-coated tablets?
- Can you take antibiotics while pregnant?
More about Erythrocin (erythromycin)
- Check interactions
- Compare alternatives
- Reviews (3)
- Side effects
- Dosage information
- During pregnancy
- Generic availability
- Drug class: macrolides
- Breastfeeding
Patient resources
Other brands
Ery-Tab, EryPed, E.E.S. Granules, Eryc, ... +2 more
Professional resources
- Erythrocin prescribing information
- Erythrocin Injection USP (FDA)
- Erythromycin Stearate (AHFS Monograph)
Other brands
EryPed, Ery-Tab, E.E.S. Granules, PCE Dispertab
Other formulations
Related treatment guides
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.