Depo-SubQ Provera 104 Dosage
Generic name: MEDROXYPROGESTERONE ACETATE 104mg in 0.65mL
Dosage form: injection, suspension
Drug classes: Contraceptives, Hormones / antineoplastics, Progestins
Medically reviewed by Drugs.com. Last updated on Jul 24, 2024.
Important Dosage and Administration Instructions
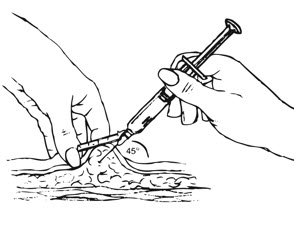
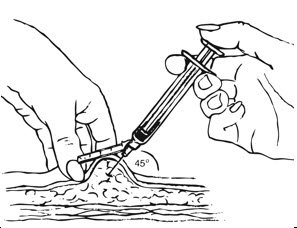
Depo-subQ provera 104 is only for subcutaneous administration and is only to be administered by a healthcare professional.
Use for longer than 2 years is not recommended (unless other birth control methods or medical therapies for endometriosis-associated pain are considered inadequate) due to the impact of long-term depo-subQ provera 104 treatment on bone mineral density (BMD).
Prior to the first injection confirm that the patient is not pregnant. For women who are sexually active and who have regular menses, administer the first injection only during the first 5 days of a normal menstrual period. For women who are breast-feeding, administer the first injection during or after the sixth post-partum week.
The recommended dosage of depo-subQ provera 104 is 104 mg given subcutaneously every 12 to 14 weeks. If more than 14 weeks elapse between injections, confirm that the patient is not pregnant before the next injection. Instruct the patient that if they are unable to receive an injection within 12–14 weeks, another contraceptive method should be used until the next depo-subQ provera 104 injection. The dosage does not need to be adjusted for body weight.
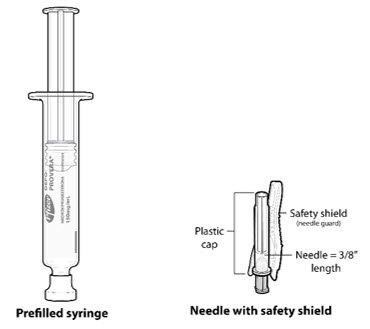
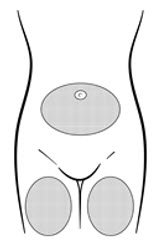
Inject the entire contents of the pre-filled syringe using strict aseptic technique into the upper anterior thigh or abdomen, rotating the sites with every injection.
Switching from Another Method of Contraception
When switching from another contraceptive method to depo-subQ provera 104, administer depo-subQ provera 104 in a manner that ensures continuous contraceptive coverage. Follow the respective recommendations when switching from the contraceptive methods listed below:
- •
- Combined hormonal contraceptives: administer the first injection of depo-subQ provera 104 within 7 days after the last day of using the combined hormonal contraceptive method (i.e., within 7 days after taking the last active pill).
- •
- An implant: administer the first injection of depo-subQ provera 104 on the day of implant removal.
- •
- A contraceptive vaginal ring or transdermal system: administer the first injection of depo-subQ provera 104 on the day the patient would have inserted the next ring or applied the next transdermal system.
- •
- An Intrauterine Device (IUD) or Intrauterine System (IUS): administer the first injection of depo-subQ provera 104 on the day of IUD/IUS removal. If the IUD/IUS is not removed on the first day of the patient's menstrual cycle, instruct patients to use a non-hormonal back-up method of birth control for the first 7 days after administration of depo-subQ provera 104.
- •
- Depot medroxyprogesterone acetate injectable suspension for intramuscular use (DMPA-IM): inject depo-subQ provera 104 12 to 14 weeks after the last dose of DMPA-IM.
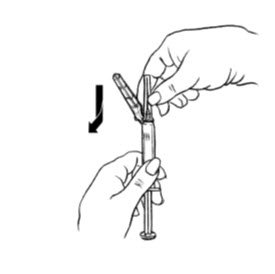
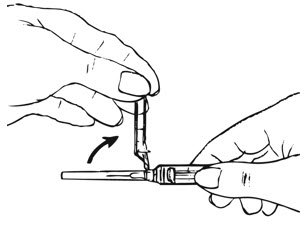
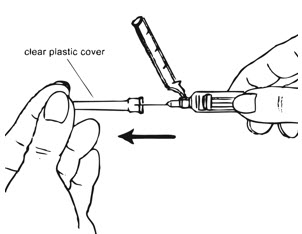
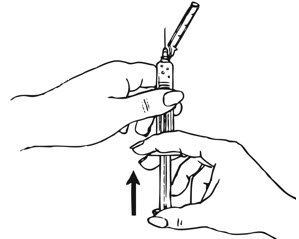
Preparation and Administration Instructions
Prior to injection:
- •
- Ensure all the components in Figure A are available and that depo-subQ provera 104 is at room temperature.
- •
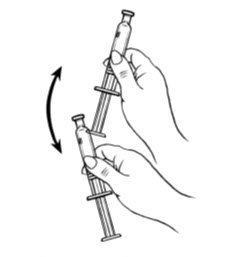
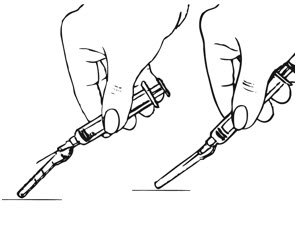
- Shake the pre-filled syringe vigorously prior to injection to ensure appropriate viscosity of the suspension.
- •
- Inspect depo-subQ provera 104 visually for particulate matter and discoloration.
Figure A. Components in the Package
Frequently asked questions
More about depo-subQ provera 104 (medroxyprogesterone)
- Check interactions
- Compare alternatives
- Pricing & coupons
- Reviews (35)
- Side effects
- During pregnancy
- FDA approval history
- Drug class: contraceptives
- Breastfeeding
- En español
Patient resources
Professional resources
Other formulations
Related treatment guides
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.