Xembify Dosage
Generic name: Human Immunoglobulin G 200mg in 1mL
Dosage form: subcutaneous injection
Drug class: Immune globulins
Medically reviewed by Drugs.com. Last updated on Feb 15, 2024.
For subcutaneous infusion only.
Before switching to XEMBIFY, obtain the patient’s serum IgG trough level to guide subsequent dose adjustments.
Dose
Individualize the dose based on the patient’s pharmacokinetic and clinical response.
Measure the patient’s serum IgG trough level as early as 5 weeks after initiating XEMBIFY treatment to determine if a dose adjustment is needed.
Monitor the patient’s IgG trough level every 2 to 3 months to determine subsequent dose adjustments and dosing intervals as needed (Table 1).
Doses divided over the course of a week or once weekly achieve similar exposure when administered regularly at steady-state.
For frequent dosing (2-7 times per week), divide the calculated weekly dose by the desired number of times per week.
For dose adjustments, calculate the difference (in mg/dL) of the patient’s serum IgG trough level from the target IgG trough level, then find this difference in Table 1 (below). Locate the corresponding amount (in mL) by which to increase or decrease the weekly dose based on the patient’s body weight. For example, if a patient with a body weight of 70 kg has an actual IgG trough level of 900 mg/dL and the target level is 1,000 mg/dL, this results in a difference of 100 mg/dL. Therefore, increase the weekly dose of subcutaneous dose by 5 mL.
The patient’s clinical response should be the primary consideration in dose adjustment. If a patient on XEMBIFY does not maintain an adequate clinical response or a serum IgG trough level equivalent to that of a previous treatment, adjust the dose accordingly.
|
|||||||||||||
| Difference From Target IgG Trough Level (mg/dL) |
Body Weight (kg) | ||||||||||||
| 10 | 15 | 20 | 30 | 40 | 50 | 60 | 70 | 80 | 90 | 100 | 110 | 120 | |
| Dose Adjustment (mL per Week)* | |||||||||||||
| 50 | 0 | 1 | 1 | 1 | 2 | 2 | 2 | 3 | 3 | 3 | 4 | 4 | 5 |
| 100 | 1 | 1 | 2 | 2 | 3 | 4 | 5 | 5 | 6 | 7 | 8 | 8 | 9 |
| 150 | 1 | 2 | 2 | 3 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 13 | 14 |
| 200 | 2 | 2 | 3 | 5 | 6 | 8 | 9 | 11 | 12 | 14 | 15 | 17 | 18 |
| 250 | 2 | 3 | 4 | 6 | 8 | 9 | 11 | 13 | 15 | 17 | 19 | 21 | 23 |
| 300 | 2 | 3 | 5 | 7 | 9 | 11 | 14 | 16 | 18 | 20 | 23 | 25 | 27 |
| 350 | 3 | 4 | 5 | 8 | 11 | 13 | 16 | 19 | 21 | 24 | 27 | 29 | 32 |
| 400 | 3 | 5 | 6 | 9 | 12 | 15 | 18 | 21 | 24 | 27 | 30 | 33 | 36 |
| 450 | 3 | 5 | 7 | 10 | 14 | 17 | 20 | 24 | 27 | 31 | 34 | 38 | 41 |
| 500 | 4 | 6 | 8 | 11 | 15 | 19 | 23 | 27 | 30 | 34 | 38 | 42 | 45 |
Switching to XEMBIFY from IVIG
Begin treatment with XEMBIFY one week after the patient’s last IVIG infusion. Calculate the initial weekly dose of XEMBIFY. Divide the previous monthly (or every 3 weeks) IVIG dose in grams by the number of weeks between IVIG infusions, then multiply this dose by the dose adjustment factor of 1.37.
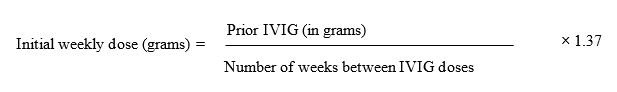
To convert the XEMBIFY dose (in grams) to milliliters (mL), multiply the calculated Initial SC dose (in grams) by 5.
Provided the total weekly dose is maintained, any dosing interval from daily up to weekly will achieve similar systemic IgG exposure when administered regularly at steady-state.
Switching to XEMBIFY from subcutaneous immune globulin (IGSC)
Administer the same weekly dose of XEMBIFY (in grams) as the weekly dose of prior IGSC treatment (in grams).
Preparation and Handling
XEMBIFY is a clear to slightly opalescent, and colorless or pale yellow solution.
Visually inspect XEMBIFY for particulate matter and discoloration prior to administration, whenever solution and container permit.
Do not use if the solution is cloudy or turbid.
Do not shake.
Do not dilute.
The XEMBIFY vial is for single use only.
Do not store any vial that has been entered by a needle during preparation for infusion, punctured, partially used, or opened.
Administer within 8 hours after beginning infusion preparation (i.e., once XEMBIFY is transferred from the vial into a syringe).
Administer XEMBIFY separately from other drugs or medications that the patient may be receiving.
Do not mix XEMBIFY with other medications including immune globulins from other manufacturers.
Do not use after expiration date.
Discard unused portion.
Administration
For subcutaneous infusion only.
Prior to use, allow the solution to reach ambient room temperature.
Do not shake.
Follow the steps below and use aseptic technique to administer XEMBIFY.
1. Inspect the vials: inspect for clarity, color, and expiration date (s).
2. Prepare for infusion:
Gather supplies: XEMBIFY vial(s), ancillary supplies, sharps container, patient’s
treatment diary/logbook, and the infusion pump.
Prepare a clean work area.
Wash hands.
3. Remove the protective cap from the vial to expose the central portion of the stopper.
If the packaging shows any sign of tampering, do not use the product and notify
Grifols Therapeutics LLC immediately [1-800-520-2807].
4. Wipe the stopper with alcohol and allow to dry.
|
 |
|
 |
|
|
|
 |
|
 |
|
 |
12. Repeat priming and needle insertion steps using a new needle, administration tubing
and a new infusion site. Secure the needle in place by applying sterile gauze or
transparent dressing over the site.
13. Infuse XEMBIFY at a maximum rate of 25 mL per hour per infusion site using up to
6 infusion sites (most patients used 4 infusion sites). Ensure that the infusion sites are
at least 2 inches (5 cm) apart for patients of all ages. The number of infusion sites is
at healthcare provider discretion. Children will require less total volume for a specific
XEMBIFY dose (mg/kg body weight) than adults. The healthcare provider may
choose a smaller volume/site for children and/or fewer infusion sites to achieve the
target total dose, depending on the needs of the child. The total dose volume of
XEMBIFY is divided by the desired volume (mL/site) to obtain number of infusion
sites to be used.
| Volume to Be Infused SC |
Rate | Number of Sites (most frequent is 4) |
Site Distance Apart |
| 25 mL per site | ≤ 25 mL/hr/infusion site | ≤ 6 | ≥ 2 inches (5 cm) |
Record information about the infusion (e.g., lot number, expiration date, dose, date,
time, infusion site location(s), side effects) in a patient treatment record or infusion
log.
14. Discard the needle(s) and infusion line(s) in an appropriate container. Follow the
manufacturer’s instructions for storage of the infusion pump.
15. Discard partially used vial(s).
More about Xembify (immune globulin subcutaneous)
- Check interactions
- Compare alternatives
- Pricing & coupons
- Latest FDA alerts (1)
- Side effects
- During pregnancy
- FDA approval history
- Drug class: immune globulins
- En español
Patient resources
- Xembify subcutaneous drug information
- Xembify (Immune globulin-klhw Subcutaneous) (Advanced Reading)
- Xembify
Other brands
Hizentra, Cuvitru, Cutaquig, Vivaglobin
Professional resources
Other brands
Related treatment guides
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.

