Tri-Solfen (Canada)
This treatment applies to the following species:Lidocaine, bupivacaine, cetrimide, and epinephrine topical solution
Veterinary Use Only
DIN 02528223
Description
Tri-Solfen is a clear, blue, semi-viscous solution containing a rapid-onset local anaesthetic, a long lasting local anaesthetic, a vasoconstrictor and an antiseptic. Each mL contains 40.6 mg of lidocaine, 4.2 mg of bupivacaine, 0.025 mg of epinephrine and 5.0 mg of cetrimide.
INDICATION
Local anaesthesia for pain mitigation during and for up to one hour after castration of piglets.
DOSAGE AND ADMINISTRATION
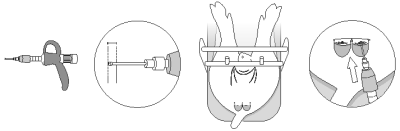
For local use only in piglets up to 7 days of age. Use a suitably calibrated applicator to apply the product.
A ball or round tipped nozzle is recommended for application to the scrotal wound.
<2 kg bw = 1 mL per piglet
2-4 kg bw = 2 mL per piglet
Set the dose at 0.5 mL. Cut the skin of the scrotum and expose the testis and spermatic cord. Via the incision, instill 1 or 2 doses (depending on the bodyweight) of 0.5 mL on either side to fully coat the exposed spermatic cords and the cut skin edges, minimizing product run off. Wait 30 seconds before severing the spermatic cord as per the standard procedure for surgical castration.
CAUTIONS
To minimize the risk of wound infection, ensure appropriate hygienic practices are followed as surgical castration is performed.
WARNINGS
Treated pigs must not be slaughtered for use in food for at least 7 days after the latest treatment with this drug.
When handling the product, avoid oral ingestion and contact with skin and eyes. Wear disposable impermeable gloves.
People with known hypersensitivity to any of the ingredients should exercise extra caution. In case of accidental skin or eye contact, wash immediately with water. Wash hands after use.
Keep out of reach of children.
ADVERSE REACTIONS
In the European clinical trial described under the efficacy studies section where 86 piglets were treated with Tri-Solfen during castration, no statistically significant difference was observed in the proportion of piglets with adverse or serious adverse events compared to the negative control group. Mild application site inflammation was the most commonly reported adverse reaction.
The frequency of adverse events and serious adverse events by treatment group in the European field trial.
|
Symptom |
Tri-Solfen |
Negative control |
|
Abdominal cavity hernia |
2 |
0 |
|
Anaphylactic shock |
1 |
0 |
|
Apathy |
1 |
2 |
|
Application site bleeding |
1 |
0 |
|
Application site inflammation |
4 |
0 |
|
Cachexia |
1 |
0 |
|
Death |
0 |
1 |
|
Diarrhea |
4 |
2 |
|
Fever |
1 |
1 |
|
Joint swelling |
0 |
1 |
|
Lameness |
1 |
1 |
|
Fibrinous enteritis |
0 |
1 |
|
Fibrinous peritonitis |
0 |
1 |
|
Pasty stool |
2 |
1 |
|
Scratching |
1 |
0 |
|
Traumatic wound/injury |
2 |
3 |
|
Vomiting |
0 |
4 |
CLINICAL PHARMACOLOGY
Lidocaine and bupivacaine are amide local anaesthetic agents which work by blocking nerve conduction. Lidocaine has a fast onset of action (less than 1 minute on open wounds and mucous membranes) with an effect lasting up to 1 hour. Bupivacaine has a slower onset of action (up to 15 minutes in open wounds), but a longer duration of action. Lidocaine in combination with bupivacaine results in an additive effect of both, with the rapid onset of action of lidocaine and the prolonged effect of bupivacaine.
Epinephrine produces vasoconstriction at the treated site, reducing bleeding, prolonging the anaesthetic effect and reducing systemic absorption of the local anaesthetics.
Cetrimide is an antiseptic (quaternary ammonium) that is used in topical wound care products and reduces bacterial colonisation of the wound.
EFFICACY STUDIES
A randomized field trial was conducted involving 173 piglets aged 3-7 days old, on two European farms. Tri-Solfen was administered during castration at the recommended label dose to 86 piglets and 87 were castrated without treatment. Effectiveness of local anaesthesia during the castration was assessed by scoring the nociceptive motor and vocal response to the procedure. The odds of piglets castrated without treatment showing pain, based on the variables mentioned above, was 2.9 times higher than those treated with Tri-Solfen (P < 0.001). Effectiveness of local anaesthesia following the castration was assessed by observing the piglets on the sow and recording acute pain-related behaviour in the 30 minutes after castration. Untreated piglets had a 2.39 times higher odds of showing a pain-related behaviour than those treated with Tri-Solfen (P < 0.0001).
A second randomized field trial was conducted involving 40 piglets aged 3-7 days of age on a farm in Australia, whereby 20 piglets were treated during castration with Tri-Solfen at the recommended label dose and the remaining piglets were treated with a placebo as they were castrated. Compared to placebo treated piglets, there was a statistically significant reduction in nociceptive motor and vocal response during castration and in wound sensitivity in the first hour following castration in Tri-Solfen treated piglets.
A field trial that enrolled 4 to 7 day-old piglets was conducted in Australia to assess the efficacy of Tri-Solfen in reducing the bacterial load at different time points following surgical castration. Thirty-six (36) piglets that underwent surgical castration were randomly allocated to one of the following treatment groups (n = 12/group): untreated, treated with Tri-Solfen or treated with a Tri-Solfen formulation that did not include cetrimide. Treatments were administered according to the recommended label dose.
The log-transformed bacterial count at one minute after castration was significantly lower for the piglets treated with Tri-Solfen than their counterparts treated with the Tri-Solfen formulation that did not include cetrimide or were not treated with any medication (1.84, 3.37 and 3.19, respectively; P < 0.05).
At 15 minutes after castration, the log-transformed bacterial count was numerically lower for the piglets treated with Tri-Solfen than their counterparts treated with the Tri-Solfen formulation that did not include cetrimide or were not treated with any medication (3.00, 3.68 and 3.85, respectively; P > 0.05). Collectively for all time points up to 4 hours after castration, the proportion of animals with a bacterial load > 105/mL was lower for the piglets treated with Tri-Solfen than their counterparts treated with the Tri-Solfen formulation that did not include cetrimide or were not treated with any medication (1.8%, 8.3% and 11.7%, respectively; P = 0.038).
SAFETY STUDIES
In a laboratory safety study in piglets, there were no drug-related adverse events and no clinically relevant changes were observed following treatment with either the recommended label dose, three or five times this dose.
No drug-related adverse events were reported in a randomized field trial that enrolled 40 piglets aged 3-7 days of age on a farm in Australia, whereby 20 piglets were treated during castration with Tri-Solfen at the recommended label dose and the remaining piglets were treated with a placebo as they were castrated.
STORAGE
Store between 15 and 25°C.
Discard 3 months after first opening.
HOW SUPPLIED
Tri-Solfen is supplied in bottles of 250 mL and 1 L
Dechra Ltd, Snaygill Industrial Estate, Keighley Road, Skipton, North Yorkshire, BD23 2RW, United Kingdom
IMPORTED AND DISTRIBUTED BY
Dechra Veterinary Products Inc., 1 Holiday Ave, East Tower, Suite 345, Pointe-Claire, Québec, H9R 5N3, Canada
DATE OF LAST REVISION
June 2022
PIL-044622-001
CPN: 1786073.0
1 HOLIDAY AVE., EAST TOWER SUITE 345, POINT-CLAIRE, QC, H9R 5N3
| Toll-Free: | 855-332-9334 | |
| Technical Services: | 855-332-9334 Option 1 | |
| Technical Services Email: | technical.ca@dechra.com | |
| Website: | www.dechra.ca |
 |
THIS SERVICE AND DATA ARE PROVIDED "AS IS". Animalytix assumes no liability, and each user assumes full risk, responsibility, and liability, related to its use of the Animalytix service and data. See the Terms of Use for further details. |

Copyright © 2024 Animalytix LLC. Updated: 2024-02-27
