Profender (emodepside/praziquantel) Topical parasiticide for medium cats (Canada)
This treatment applies to the following species: Company: Vetoquinol
Company: Vetoquinol
DIN 02316137
Topical parasiticide for small cats: 7.5 mg emodepside and 30 mg praziquantel
DIN 02316145
Topical parasiticide for medium cats: 15 mg emodepside and 60 mg praziquantel
DIN 02316153
Topical parasiticide for large cats: 24 mg emodepside and 96 mg praziquantel
VETERINARY USE ONLY
Description
profender, a topical parasiticide containing emodepside and praziquantel, is a clear yellow to brownish ready-to-use solution packaged in unit dosing applicator tubes for treatment of cats and kittens 8 weeks of age and older. The formulation and dosage schedule is designed to provide a minimum of 3 mg of emodepside and 12 mg of praziquantel per kg of body weight.
Profender (emodepside/praziquantel) Topical parasiticide for medium cats Indications
profender topical parasiticide is indicated in cats and kittens, 8 weeks of age and older, for the treatment and control of parasitic infections caused by the developing L4, the sexually immature adult and the adult stages of hookworms (Ancylostoma tubaeforme) and ascarids (Toxocara cati) and all intestinal stages of tapeworms (Dipylidium caninum and Taenia taeniaeformis).
DOSAGE:
Apply to the skin at the recommended minimum dose of 3 mg of emodepside and 12 mg of praziquantel per kg of body weight, equivalent to 0.14 mL profender topical parasiticide per kg of body weight.
|
Body Weight of Cat (kg) |
Pipette size to be used |
Volume (mL) |
Emodepside |
Praziquantel |
|
≥0.5 - 2.5 |
profender for Small Cats |
0.35 |
3 - 15 |
12 - 60 |
|
>2.5 - 5 |
profender for Medium Cats |
0.70 |
3 - 6 |
12 - 24 |
|
>5 - 8 |
profender for Large Cats |
1.12 |
3 - 4.8 |
12 - 19.2 |
*Cats over 8 kg should be treated with the appropriate combination of profender topical parasiticide tubes.
Frequency of dosing: For the treatment of roundworm (ascarids), hookworm and tapeworm infections, a single dose is effective. In cats where re-infection is common, control can be obtained with monthly application of profender, or as directed by a veterinarian.
ADMINISTRATION:
Remove one pipette from package. Hold pipette in upright position, twist and pull off cap and use the opposite end of the cap to break the seal.
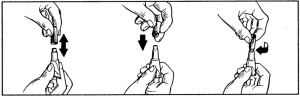
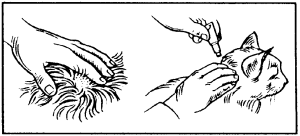
Apply only to the skin surface and on intact skin. Part the fur on the cat’s neck at the base of the skull until the skin is visible. Place the tip of the pipette on the skin and squeeze firmly several times to empty the contents directly onto the skin. Application on the base of the skull will minimize the ability of the cat to lick the product off.
Treatment and Control of Intestinal Nematode and Cestode Infections:
profender will treat parasitic infections due to the developing L4, the sexually immature adult and the adult stages of hookworms (Ancylostoma tubaeforme) and ascarids (Toxocara cati) and all intestinal stages of tapeworms (Dipylidium caninum and Taenia taeniaeformis). Monthly administration of profender, or as directed by a veterinarian, will control the development of these intestinal nematodes and cestodes in cats.
CAUTIONS:
Use with caution in sick, debilitated, or underweight animals. Do not use in kittens under 8 weeks of age or weighing less than 0.5 kg. For topical use only.
Salivation and vomiting may occasionally occur. This is thought to occur as a result of the cat licking the application site immediately after treatment. Emodepside is a substrate for P-glycoprotein. Co-treatment with other drugs that are P-glycoprotein substrates/inhibitors (for example, other antiparasitic macrocyclic lactones, erythromycin, prednisolone and cyclosporine) could give rise to pharmacokinetic drug interactions. The potential clinical consequences of such interactions have not been investigated. The solvent in this product may stain certain materials including leather, fabrics, plastics and finished surfaces. Allow the application site to dry before permitting contact with such materials. The reproductive safety of profender topical parasiticide has not been demonstrated in male breeding cats.
Warnings
Keep out of reach of children. May be irritating to skin and eyes. If contact with eyes occurs, flush eyes copiously with water. If eye irritation persists, contact a physician. Wash hands after use. Avoid direct contact with application area while it is wet. If contact with skin occurs, wash skin immediately with soap and water.
If ingested, contact a physician immediately.
Adverse Reactions
Salivation and vomiting may occur in very rare cases. Mild and transient neurological disorders such as ataxia or tremor may occur in very rare cases. These effects are thought to occur as a result of the cat licking the application site immediately after treatment. In very rare cases following administration of profender, transient alopecia, pruritus and/or inflammation were observed at the application site.
Post-Market Experience:
Although all adverse reactions are not reported, the following information is based on voluntary post-approval drug experience reporting. It is generally recognized that this results in significant under-reporting. The adverse events listed here reflect reporting and not causality. Adverse events are listed by body system, in decreasing order or frequency:
Digestive tract disorders: vomiting, hypersalivation
Application site disorders: alopecia, pruritus, inflammation
Systemic disorders: lethargy, anorexia,
Neurological disorders: ataxia, muscle tremor
To report suspected adverse drug events or for technical assistance, contact Vetoquinol N.-A. Inc. at 1 800 363-1700.
Overdose: Salivation, vomiting and neurological signs were observed occasionally when the product was administered at up to 10 times the recommended dose in adult cats and up to 5 times the recommended dose in kittens. These symptoms were thought to occur as a result of the cat licking the application site. The symptoms were completely reversible in these studies. There is no known specific antidote.
PHARMACODYNAMICS:
Emodepside is a semi-synthetic compound belonging to the new chemical group of depsipeptides. It is active against roundworms (ascarids and hookworms). In this product, emodepside is responsible for the efficacy against Toxocara cati and Ancylostoma tubaeforme. It acts at the neuromuscular junction by stimulating presynaptic receptors belonging to the secretin receptor family which results in paralysis and death of the parasites.
Praziquantel is a pyrazinoisoquinoline derivative effective against tapeworms such as Dipylidium caninum and Taenia taeniaeformis.
Praziquantel is rapidly adsorbed via the surface of the parasites and acts primarily by changing the Ca++ permeability of the parasite membranes. This results in severe damage to the parasite integument, contraction and paralysis, disruption of metabolism and finally leads to the death of the parasite.
PHARMACOKINETICS:
After topical application of this product to cats at the minimum therapeutic dose of 0.14 mL/kg bodyweight, mean maximum serum concentrations of 32.2 ± 23.9 µg emodepside/L and 61.3 ± 44.1 µg praziquantel/L were observed. Maximum concentrations were reached for emodepside 3.2 ± 2.7 days after application and 18.7 ± 47 hours for praziquantel. Both active substances are then slowly eliminated from the serum with a half-life of 9.2 ± 3.9 days for emodepside and 4.1 ± 1.5 days for praziquantel. After oral application in the rat, emodepside is distributed to all organs. Highest concentration levels are found in the fat. Faecal excretion predominates with unchanged emodepside and hydroxylated derivatives as the major excretion products.
Studies in many different species show that praziquantel is rapidly metabolised in the liver. The main metabolites are monohydroxycyclohexyl derivatives of praziquantel. Renal elimination predominates.
Animal Safety
A significant safety margin was demonstrated for dermal overdoses of profender in kittens (up to 5 times the maximum dose a kitten would receive in the field) and adult cats (10 times the recommended unit dosage). The clinical signs which were observed can all be attributed to oral uptake of the product. Although it was attempted in all of the target animal safety studies to apply the product in a manner that the cats could not easily reach the application site it was not possible to prevent them from oral uptake by grooming and licking the application site when high doses were applied, especially in kittens. The clinical signs which were associated with oral exposure were generally mild and reversible and included salivation and vomiting. In rare cases mild tremors were observed in cats which were treated with high overdoses. Similarly, direct oral application of the dermal therapeutic dose to adult cats caused transient salivation and vomiting in a few animals. These are normal defence mechanisms to an oral irritant, therefore these clinical signs were not unexpected.
Safety in Pregnant and Lactating Queens:
A study was conducted to investigate the safety of profender when applied during pregnancy and lactation. profender was administered three times during pregnancy and three times during lactation at one (1x) or three (3x) times the maximum therapeutic dose for medium sized cats and compared with a treatment with tap water. No local or general clinical signs associated with the treatment were observed in any of the queens and no clinical signs which could be related to the treatment given to the mothers were observed in the kittens. There were no biologically relevant differences between groups with regard to body weight, haematology and clinical chemistry of queens and kittens. Gestation rates, mean gestation length, litter size, viability index and the ratio of male to female kittens and percentage of postnatal losses were considered to be unaffected by the treatment. It was concluded that there was no effect of treatment of queens on mothering and on kitten viability. All 89 kittens born were free of externally visible defects. Thus it was concluded that there was no indication of a fetotoxic or teratogenic potential. The results of this study demonstrated a significant margin of safety for the administration of profender during pregnancy and lactation.
profender was safe in pregnant and lactating queens and their progeny.
There is no evidence for a breed sensitivity or an incompatibility with concomitantly applied treatments. After use of the product within the scope of the pharmacokinetic and the efficacy studies very few clinical signs were observed.
Storage
Store at or below 30°C. Protect from freezing.How Supplied
|
Applicator Tube Size |
Applications per Package |
|
0.35 mL |
40 - 0.35 mL tubes (10 blisters of 4 tubes) |
|
0.35 mL |
2 - 0.35 mL tubes (1 blister of 2 tubes) |
|
0.70 mL |
40 - 0.70 mL tubes (10 blisters of 4 tubes) |
|
0.70 mL |
2 - 0.70 mL tubes (1 blister of 2 tubes) |
|
1.12 mL |
24 - 1.12 mL tubes (6 blisters of 4 tubes) |
|
1.12 mL |
2 - 1.12 mL tubes (1 blister of 2 tubes) |
(Not all packaging sizes may be marketed.)
®Registered trademark of Vetoquinol N.-A. Inc.
Vetoquinol N.-A. Inc., 2000, ch. Georges, Lavaltrie, QC, Canada J5T 3S5
0521A
CPN: 1234481.1
Commercial Division
2000, CHEMIN GEORGES, LAVALTRIE, QC, J5T 3S5
| Telephone: | 450-586-2252 | |
| Order Desk: | 800-363-1700 | |
| Fax: | 450-586-4649 | |
| Website: | www.vetoquinol.ca | |
| Email: | info@vetoquinol.ca |
 |
THIS SERVICE AND DATA ARE PROVIDED "AS IS". Animalytix assumes no liability, and each user assumes full risk, responsibility, and liability, related to its use of the Animalytix service and data. See the Terms of Use for further details. |

Copyright © 2024 Animalytix LLC. Updated: 2024-02-27
