PRID DELTA (Canada)
This treatment applies to the following species: Company: Ceva Animal Health
Company: Ceva Animal Health
progesterone releasing device
DIN 02297507
VETERINARY USE ONLY
Description
PRID® DELTA is an intravaginal progesterone releasing device made of ethyl vinyl acetate and polyamide which contains 1.55 g of progesterone.
ACTION: Progesterone is a naturally occurring hormone produced by the corpus luteum during diestrus in non-pregnant animals, and by the corpus luteum and the placenta during pregnancy. Under the influence of progesterone, normal pituitary gonadotrophin output is inhibited and the ovarian cycle is interrupted. When the PRID® DELTA is inserted into the vagina of dairy cows, progesterone is slowly released over the 7 day treatment period. The removal of the PRID® DELTA results in the rapid decline of plasma progesterone and the onset of estrus in animals responding to treatment.
PRID DELTA Indications
For the treatment of anestrus in dairy cows greater than 60 days post-partum.
Directions For Use
Using the special applicator, insert one (1) PRID® DELTA into the vagina of each cow to be treated for a period of 7 days. After 7 days, remove the PRID® DELTA and inject a luteolytic dose of prostaglandin F2α. Cows responding to treatment will come into estrus within 1 to 5 days. Inseminate when estrus is detected, as per the routine of the herd.
● Preparing to Insert
It is recommended that one bucket be filled with a non-irritating disinfectant solution and another bucket be filled with clean water. Wash the applicator in the disinfecting solution and rinse in cold water prior to and after each use. As a further precaution, cold sterilise the applicator between herds if it is to be used on more than one herd.
Restrain animal appropriately.
Wear gloves when handling PRID® DELTA.
Clean and disinfect the applicator before and after use and between each animal.
● Insertion
1. Check the orientation of the device.
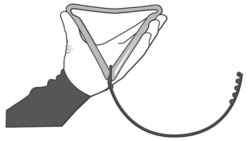
2. Fold the PRID® DELTA before inserting into the applicator.
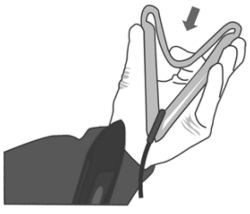
3. Push the device into the applicator up to the end.
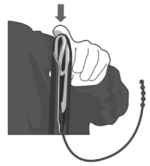
4. Apply a veterinary obstetrical lubricant to the outside of the applicator.
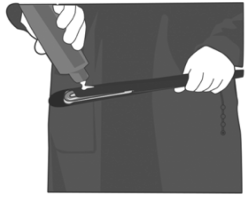
5. Lift the cow’s tail. The exterior of the vulva should be washed with a disinfecting solution and dried. Gently insert the applicator in the vagina, holding it by its handle. Place the device as far as possible without forcing. Release the device by pushing the handle.
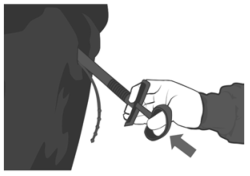
6. The string attached to the PRID® DELTA should be visible from the vulva.
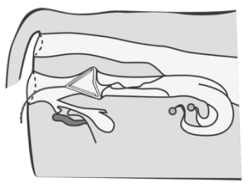
● Removal
Remove the device 7 days post insertion by gently pulling the exposed string.
Note: The expected retention rate is 98%. If loss rates exceed 2%, re-evaluate insertion technique.
● Timing for insemination
It is recommended that animals are inseminated at observed heat. Animals which lose the PRID® DELTA during the treatment period should be bred at next observed estrus.
Contraindications
Do not use in dairy cows with abnormal or infected reproductive tracts.
CAUTIONS:
● Safety and efficacy of this drug have not been evaluated in dairy heifers prior to first calving or in dairy cows less than 60 days post-partum.
● Contact a veterinarian if abnormal bloody discharge is observed during treatment.
● Do not reuse the device.
● Care must be taken when using the applicator so as not to damage the vagina.
● Do not use if the device is damaged.
● Animals in poor condition resulting from illness or nutritional stress may not respond to this drug.
Warnings
KEEP OUT OF REACH OF CHILDREN. Gloves should be worn when handling the PRID® DELTA. Remove PRID® DELTA before sending treated animals for slaughter.Adverse Reactions
Mucopurulent vaginal discharge, vaginal inflammation, as well as an offensive odour, may be observed at the time of removal of the PRID® DELTA. These adverse events are caused by the presence of the PRID® DELTA and generally resolve after removal.Pivotal Target Animal Safety And Efficacy Studies
EFFICACY: Effectiveness of the PRID® DELTA was confirmed using plasma bioequivalence data, comparing the redesigned product to the reference product, PRID®. A multi-location field study was conducted to evaluate the efficacy of the Progesterone Releasing Intravaginal Device to treat anestrus in dairy cows greater than 60 days postpartum. Heifers were not included in this study. Holstein cows (n=410) which had not displayed signs of estrus and were greater than 60 days postpartum were assigned to receive either progesterone (n=218) or a placebo (device without progesterone) (n=192). Devices were inserted for 7 days. After 7 days, devices were removed and prostaglandin F2α was administered intramuscularly. Cows were detected in estrus by pedometry and inseminated at estrus according to the normal routine of the herd. In animals receiving and retaining the device for the 7 day treatment period 60.5% of progesterone treated cows showed estrus within 5 days of device removal and prostaglandin injection, versus 38.0% in the placebo group. The median time from device insertion to insemination was 12 days for progesterone treated cows versus 23 days in cows treated with the placebo.
SAFETY: The effect of PRID® DELTA on vaginal secretion and inflammation was studied on thirty cows treated for a 7-day period with PRID® DELTA. Local tolerance was observed for the treatment period and 6 days after the withdrawal of the device. Local reactions were recorded and classified with gradation from level 1 (weak reaction) to level 4 (very important reaction).
The device stayed in place in the vagina for the 7-day treatment period and no loss was recorded.
The presence of the device in the vagina led to the production of non-transparent vaginal secretions and to vaginal inflammation. Most of the recorded reactions were graded level 1 or level 2 and were considered to be weak or moderate reactions. Important or very important vaginal inflammation were observed at day 7, at the time of vaginal examination at the removal of the device, affecting 16.7% of the treated cows and were not recorded at day 9.
Production of non-transparent vaginal secretions were recorded from day 3 to day 7 in 53.3 to 66.7% of the treated cows respectively, with a maximum frequency at day 5 in 70% of the treated cows. This production was not very abundant, and decreased and disappeared spontaneously throughout the observed period. Vaginal inflammation was more frequently observed in the treated cows at the time of removal of the device and decreased and disappeared spontaneously within days after removal.
Regarding the different studies performed with PRID® DELTA, approximately 1.6% of animals (1/61) lost the device during the 7 day treatment period.
STORAGE CONDITIONS: Store between 15-30°C. Protect from freezing.
®PRID DELTA is a registered trademark of Ceva Santé Animale, France
Ceva Animal Health Inc., 6-1040 Fountain St. N. Cambridge, ON, Canada N3E 1A3
1-800-510-8864
C614-3120202
Last Revised: 2017-12-05
Presentation: 10 devices per box.
CPN: 1221134.0
150 RESEARCH LANE, SUITE 225, GUELPH, ON, N1G 4T2
| Telephone: | 519-650-9570 | |
| Toll-Free: | 800-510-8864 | |
| Fax: | 519-650-9576 | |
| Website: | www.ceva-canada.ca | |
| Email: | service.canada@ceva.com |
 |
THIS SERVICE AND DATA ARE PROVIDED "AS IS". Animalytix assumes no liability, and each user assumes full risk, responsibility, and liability, related to its use of the Animalytix service and data. See the Terms of Use for further details. |

Copyright © 2024 Animalytix LLC. Updated: 2024-02-27
