The originating document has been archived. We cannot confirm the completeness, accuracy, or currency of the content.
PREVICOX Chewable Tablets
This page contains information on PREVICOX Chewable Tablets for veterinary use.The information provided typically includes the following:
- PREVICOX Chewable Tablets Indications
- Warnings and cautions for PREVICOX Chewable Tablets
- Direction and dosage information for PREVICOX Chewable Tablets
PREVICOX Chewable Tablets
This treatment applies to the following species: Company: Merial
Company: Merial
(firocoxib)
For oral use in dogs only.
Caution: Federal law restricts this drug to use by or on the order of a licensed veterinarian.
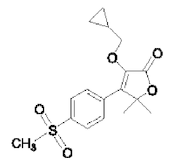
Description: PREVICOX (firocoxib) belongs to the coxib class of non-narcotic, non-steroidal anti-inflammatory drugs. Firocoxib is a white crystalline compound described chemically as 3-(cyclopropylmethoxy)-4-(4-(methylsulfonyl)phenyl)-5,5-dimethylfuranone. The empirical formula is C17H20O5S, and the molecular weight is 336.4. The structural formula is shown below:
Pharmacokinetics: The absolute bioavailability of PREVICOX (firocoxib) is approximately 38% when administered as a 5 mg/kg oral dose to fasted adult dogs. Firocoxib is rapidly cleared from the blood via hepatic metabolism and fecal excretion (CLsystemic = ~0.4 L/hr/kg). Despite a high level of plasma protein binding (96%), firocoxib exhibits a large volume of distribution (Vdλ of total drug = ~4.6 L/kg) and a terminal elimination half life of 7.8 hours (%CV = 30%). The oral drug absorption process is highly variable among subjects. Co-administration of PREVICOX with food delays drug absorption (Tmax from 1 to 5 hours) and decreases peak concentrations (Cmax from 1.3 to 0.9 mcg/mL). However, food does not affect the overall oral bioavailability at the recommended dose.
Indications: PREVICOX (firocoxib) Chewable Tablets are indicated for the control of pain and inflammation associated with osteoarthritis and for the control of postoperative pain and inflammation associated with soft-tissue and orthopedic surgery in dogs.
Dosage and Administration: Always provide Client Information Sheet with prescription. Carefully consider the potential benefits and risks of PREVICOX and other treatment options before deciding to use PREVICOX. Use the lowest effective dose for the shortest duration consistent with individual response. The recommended dosage of PREVICOX (firocoxib) for oral administration in dogs is 2.27 mg/lb (5.0 mg/kg) body weight once daily as needed for osteoarthritis and for 3 days as needed for postoperative pain and inflammation associated with soft-tissue and orthopedic surgery. The dogs can be treated with PREVICOX approximately two hours prior to surgery. The tablets are scored and dosage should be calculated in half tablet increments. PREVICOX Chewable Tablets can be administered with or without food.
Contraindications: Dogs with known hypersensitivity to firocoxib should not receive PREVICOX.
Warnings: Not for use in humans. Keep this and all medications out of the reach of children. Consult a physician in case of accidental ingestion by humans.
For oral use in dogs only. Use of this product at doses above the recommended 2.27 mg/lb (5.0 mg/kg) in puppies less than seven months of age has been associated with serious adverse reactions, including death (see Animal Safety). Due to tablet sizes and scoring, dogs weighing less than 12.5 lb (5.7 kg) cannot be accurately dosed.
All dogs should undergo a thorough history and physical examination before the initiation of NSAID therapy. Appropriate laboratory testing to establish hematological and serum baseline data is recommended prior to and periodically during administration of any NSAID. Owners should be advised to observe for signs of potential drug toxicity (see Adverse Reactions and Animal Safety) and be given a Client Information Sheet about PREVICOX Chewable Tablets.
For technical assistance or to report suspected adverse events, call 1-877-217-3543.
Precautions: This product cannot be dosed accurately in dogs less than 12.5 pounds in body weight.
Consider appropriate washout times when switching from one NSAID to another or when switching from corticosteroid use to NSAID use.
As a class, cyclooxygenase inhibitory NSAIDs may be associated with renal, gastrointestinal and hepatic toxicity. Sensitivity to drug-associated adverse events varies with the individual patient. Dogs that have experienced adverse reactions from one NSAID may experience adverse reactions from another NSAID. Patients at greatest risk for adverse events are those that are dehydrated, on concomitant diuretic therapy, or those with existing renal, cardiovascular, and/or hepatic dysfunction. Concurrent administration of potentially nephrotoxic drugs should be carefully approached and monitored. NSAIDs may inhibit the prostaglandins that maintain normal homeostatic function. Such anti-prostaglandin effects may result in clinically significant disease in patients with underlying or pre-existing disease that has not been previously diagnosed. Since NSAIDs possess the potential to produce gastrointestinal ulcerations and/or gastrointestinal perforations, concomitant use with other anti-inflammatory drugs, such as NSAIDs or corticosteroids, should be avoided. The concomitant use of protein bound drugs with PREVICOX Chewable Tablets has not been studied in dogs. Commonly used protein-bound drugs include cardiac, anticonvulsant, and behavioral medications. The influence of concomitant drugs that may inhibit the metabolism of PREVICOX Chewable Tablets has not been evaluated. Drug compatibility should be monitored in patients requiring adjunctive therapy.
If additional pain medication is needed after the daily dose of PREVICOX, a non-NSAID class of analgesic may be necessary.
Appropriate monitoring procedures should be employed during all surgical procedures. Anesthetic drugs may affect renal perfusion, approach concomitant use of anesthetics and NSAIDs cautiously. The use of parenteral fluids during surgery should be considered to decrease potential renal complications when using NSAIDs perioperatively.
The safe use of PREVICOX Chewable Tablets in pregnant, lactating or breeding dogs has not been evaluated.
Adverse Reactions:
Osteoarthritis: In controlled field studies, 128 dogs (ages 11 months to 15 years) were evaluated for safety when given PREVICOX Chewable Tablets at a dose of 2.27 mg/lb (5.0 mg/kg) orally once daily for 30 days. The following adverse reactions were observed. Dogs may have experienced more than one of the observed adverse reactions during the study.
Adverse Reactions Seen in U.S. Field Studies
|
Adverse Reactions |
Previcox |
Active Control |
|
Vomiting |
5 |
8 |
|
Diarrhea |
1 |
10 |
|
Decreased Appetite or Anorexia |
3 |
3 |
|
Lethargy |
1 |
3 |
|
Pain |
2 |
1 |
|
Somnolence |
1 |
1 |
|
Hyperactivity |
1 |
0 |
PREVICOX (firocoxib) Chewable Tablets were safely used during field studies concomitantly with other therapies, including vaccines, anthelmintics, and antibiotics.
Soft-tissue Surgery: In controlled field studies evaluating soft-tissue postoperative pain and inflammation, 258 dogs (ages 10.5 weeks to 16 years) were evaluated for safety when given PREVICOX Chewable Tablets at a dose of 2.27 mg/lb (5.0 mg/kg) orally approximately 2 hours prior to surgery and once daily thereafter for up to two days. The following adverse reactions were observed. Dogs may have experienced more than one of the observed reactions during the study.
Adverse Reactions Seen in the Soft-tissue Surgery Postoperative Pain Field Studies
|
Adverse Reactions |
Firocoxib Group |
Control Group* |
|
Vomiting |
5 |
6 |
|
Diarrhea |
1 |
1 |
|
Bruising at Surgery Site |
1 |
1 |
|
Respiratory Arrest |
1 |
0 |
|
SQ Crepitus in Rear Leg and Flank |
1 |
0 |
|
Swollen Paw |
1 |
0 |
*Sham-dosed (pilled)
Orthopedic Surgery: In a controlled field study evaluating orthopedic postoperative pain and inflammation, 226 dogs of various breeds, ranging in age from 1 to 11.9 years in the PREVICOX-treated groups and 0.7 to 17 years in the control group were evaluated for safety. Of the 226 dogs, 118 were given PREVICOX Chewable Tablets at a dose of 2.27 mg/lb (5.0 mg/kg) orally approximately 2 hours prior to surgery and once daily thereafter for a total of three days. The following adverse reactions were observed. Dogs may have experienced more than one of the observed reactions during the study.
Adverse Reactions Seen in the Orthopedic Surgery Postoperative Pain Field Study
|
Adverse Reactions |
Firocoxib Group |
Control Group* |
|
Vomiting |
1 |
0 |
|
Diarrhea |
2** |
1 |
|
Bruising at Surgery Site |
2 |
3 |
|
Inappetence/Decreased Appetite |
1 |
2 |
|
Pyrexia |
0 |
1 |
|
Incision Swelling, Redness |
9 |
5 |
|
Oozing Incision |
2 |
0 |
A case may be represented in more than one category.
*Sham-dosed (pilled).
**One dog had hemorrhagic gastroenteritis.
Post Approval Experience: The following adverse reactions are based on voluntary post-approval reporting and are consistent with those reported for other cyclooxygenase inhibitory NSAID class drugs. The categories are listed in decreasing order of frequency by body system.
Gastrointestinal: Vomiting, anorexia, diarrhea, melena, hematemesis, hematochezia, weight loss, nausea, gastrointestinal ulceration, gastrointestinal perforation, salivation.
Urinary: Azotemia, elevated creatinine, polydipsia, polyuria, urinary tract infection, hematuria, urinary incontinence, renal failure.
Hematological: Anemia, thrombocytopenia.
Hepatic: Hepatic enzyme elevations, decreased or increased total protein and globulin, decreased albumin, decreased BUN, icterus, ascites, pancreatitis.
Neurological / Behavioral / Special Sense: Lethargy, weakness, seizure, ataxia, aggression, tremor, uveitis, mydriasis, nystagmus.
Cardiovascular / Respiratory: Tachypnea.
Dermatological / Immunological: Fever, facial / muzzle edema, pruritus, urticaria, moist dermatitis.
In rare situations, death has been reported as an outcome of the adverse events listed above.
Information For Dog Owners: PREVICOX, like other drugs of its class, is not free from adverse reactions. Owners should be advised of the potential for adverse reactions and be informed of the clinical signs associated with drug intolerance. Adverse reactions may include vomiting, diarrhea, decreased appetite, dark or tarry stools, increased water consumption, increased urination, pale gums due to anemia, yellowing of gums, skin or white of the eye due to jaundice, lethargy, incoordination, seizure, or behavioral changes. Serious adverse reactions associated with this drug class can occur without warning and in rare situations result in death (see Adverse Reactions). Owners should be advised to discontinue PREVICOX therapy and contact their veterinarian immediately if signs of intolerance are observed. The vast majority of patients with drug related adverse reactions have recovered when the signs are recognized, the drug is withdrawn, and veterinary care, if appropriate, is initiated. Owners should be advised of the importance of periodic follow up for all dogs during administration of any NSAID.
Clinical Pharmacology: Mode of action: PREVICOX (firocoxib) is a cyclooxygenase-inhibiting (coxib) class, non-narcotic, non-steroidal anti-inflammatory drug (NSAID) with anti-inflammatory and analgesic properties. There are two main cyclooxygenase enzymes, COX-1 and COX-2, and a newly discovered third enzyme, COX-3, which has yet to be fully characterized.1 Cyclooxygenase-1 (COX-1) is the enzyme responsible for facilitating constitutive physiologic processes, e.g., platelet aggregation, gastric mucosal protection, and renal perfusion.2 It also is constitutively expressed in the brain, spinal cord, and reproductive tract.3 Cyclooxygenase-2 (COX-2) is responsible for the synthesis of inflammatory mediators, but it is also constitutively expressed in the brain, spinal cord and kidneys.4,5,6 Cyclooxygenase-3 (COX-3) is also constitutively expressed in the canine and human brain and also the human heart.7 Results from in vitro studies showed firocoxib to be highly selective for the COX-2 enzyme when canine blood was exposed to drug concentrations comparable to those observed following a once daily 5 mg/kg oral dose in dogs.8 However, the clinical significance of these findings has not been established.
Effectiveness: Two hundred and forty-nine dogs of various breeds, ranging in age from 11 months to 20 years, and weighing 13 to 175 lbs, were randomly administered PREVICOX or an active control drug in two field studies. Dogs were assessed for lameness, pain on manipulation, range of motion, joint swelling, and overall improvement in a non-inferiority evaluation of PREVICOX compared with the active control. At the study’s end, 87% of the owners rated PREVICOX-treated dogs as improved. Eighty-eight percent of dogs treated with PREVICOX were also judged improved by the veterinarians. Dogs treated with PREVICOX showed a level of improvement in veterinarian-assessed lameness, pain on palpation, range of motion, and owner-assessed improvement that was comparable to the active control. The level of improvement in PREVICOX-treated dogs in limb weight bearing on the force plate gait analysis assessment was comparable to the active control.
In a separate field study, two hundred fifty-eight client-owned dogs of various breeds, ranging in age from 10.5 weeks to 16 years and weighing from 7 to 168 lbs, were randomly administered PREVICOX or a control (sham-dosed-pilled) for the control of postoperative pain and inflammation associated with soft-tissue surgical procedures such as abdominal surgery (e.g. ovariohysterectomy, abdominal cryptorchidectomy, splenectomy, cystotomy) or major external surgeries (e.g. mastectomy, skin tumor removal ≥8 cm). The study demonstrated that PREVICOX treated dogs had significantly lower need for rescue medication than the control (sham-dosed-pilled) in controlling postoperative pain and inflammation associated with soft-surgery.
A multi-center field study with 226 client-owned dogs of various breeds, and ranging in age from 1 to 11.9 years in the PREVICOX-treated groups and 0.7 to 17 years in the control group was conducted. Dogs were randomly assigned to either the PREVICOX or the control (sham-dosed-pilled) group for the control of postoperative pain and inflammation associated with orthopedic surgery. Surgery to repair a ruptured cruciate ligament included the following stabilization procedures: fabellar suture and/or imbrication, fibular head transposition, tibial plateau leveling osteotomy (TPLO), and ‘over the top’ technique. The study (n = 220 for effectiveness) demonstrated that PREVICOX-treated dogs had significantly lower need for rescue medication than the control (sham-dosed-pilled) in controlling postoperative pain and inflammation associated with orthopedic surgery.
Palatability: PREVICOX Chewable Tablets were rated both convenient to administer (97.2%) and palatable to the dog (68.5%) by owners in multi-center field studies involving client-owned dogs of various breeds and sizes.
Animal Safety: In a target animal safety study, firocoxib was administered orally to healthy adult Beagle dogs (eight dogs per group) at 5, 15, and 25 mg/kg (1, 3, and 5 times the recommended total daily dose) for 180 days. At the indicated dose of 5 mg/kg, there were no treatment related adverse events. Decreased appetite, vomiting, and diarrhea were seen in dogs in all dose groups, including unmedicated controls, although vomiting and diarrhea were seen more often in dogs in the 5X dose group. One dog in the 3X dose group was diagnosed with juvenile polyarteritis of unknown etiology after exhibiting recurrent episodes of vomiting and diarrhea, lethargy, pain, anorexia, ataxia, proprioceptive deficits, decreased albumin levels, decreased and then elevated platelet counts, increased bleeding times, and elevated liver enzymes. On histopathologic examination, a mild ileal ulcer was found in one 5X dog. This dog also had a decreased serum albumin which returned to normal by study completion. One control and three 5X dogs had focal areas of inflammation in the pylorus or small intestine. Vacuolization without inflammatory cell infiltrates was noted in the thalamic region of the brain in three control, one 3X, and three 5X dogs. Mean ALP was within the normal range for all groups but was greater in the 3X and 5X dose groups than in the control group. Transient decreases in serum albumin were seen in multiple animals in the 3X and 5X dose groups, and in one control animal.
In a separate safety study, firocoxib was administered orally to healthy juvenile (10-13 weeks of age) Beagle dogs at 5, 15, and 25 mg/kg (1, 3, and 5 times the recommended total daily dose) for 180 days. At the indicated (1X) dose of 5 mg/kg, on histopathologic examination, three out of six dogs had minimal periportal hepatic fatty change. On histopathologic examination, one control, one 1X, and two 5X dogs had diffuse slight hepatic fatty change. These animals showed no clinical signs and had no liver enzyme elevations. In the 3X dose group, one dog was euthanized because of poor clinical condition (Day 63). This dog also had a mildly decreased serum albumin. At study completion, out of five surviving and clinically normal 3X dogs, three had minimal periportal hepatic fatty change. Of twelve dogs in the 5X dose group, one died (Day 82) and three moribund dogs were euthanized (Days 38, 78, and 79) because of anorexia, poor weight gain, depression, and in one dog, vomiting. One of the euthanized dogs had ingested a rope toy. Two of these 5X dogs had mildly elevated liver enzymes. At necropsy all five of the dogs that died or were euthanized had moderate periportal or severe panzonal hepatic fatty change; two had duodenal ulceration; and two had pancreatic edema. Of two other clinically normal 5X dogs (out of four euthanized as comparators to the clinically affected dogs), one had slight and one had moderate periportal hepatic fatty change. Drug treatment was discontinued for four dogs in the 5X group. These dogs survived the remaining 14 weeks of the study. On average, the dogs in the 3X and 5X dose groups did not gain as much weight as control dogs. Rate of weight gain was measured (instead of weight loss) because these were young growing dogs. Thalamic vacuolation was seen in three of six dogs in the 3X dose group, five of twelve dogs in the 5X dose group, and to a lesser degree in two unmedicated controls. Diarrhea was seen in all dose groups, including unmedicated controls.
In a separate dose tolerance safety study involving a total of six dogs (two control dogs and four treated dogs), firocoxib was administered to four healthy adult Beagle dogs at 50 mg/kg (ten times the recommended daily dose) for twenty-two days. All dogs survived to the end of the study. Three of the four treated dogs developed small intestinal erosion or ulceration. Treated dogs that developed small intestinal erosion or ulceration had a higher incidence of vomiting, diarrhea, and decreased food consumption than control dogs. One of these dogs had severe duodenal ulceration, with hepatic fatty change and associated vomiting, diarrhea, anorexia, weight loss, ketonuria, and mild elevations in AST and ALT. All four treated dogs exhibited progressively decreasing serum albumin that, with the exception of one dog that developed hypoalbuminemia, remained within normal range. Mild weight loss also occurred in the treated group. One of the two control dogs and three of the four treated dogs exhibited transient increases in ALP that remained within normal range.
Storage: Store at room temperature, between 59°-86° F (15°-30° C). Brief periods up to 104° F (40° C) are permitted.
To Request a Material Safety Data Sheet (MSDS), call 1-877-217-3543.
How Supplied: PREVICOX is available as round, beige to tan, half-scored tablets in two strengths, containing 57 mg or 227 mg firocoxib. Each tablet strength is supplied in 3 count, 10 count and 30 count blister packages and 60 count bottles.
1 Willoughby DA, Moore AR and Colville-Nash PR. COX-1, COX-2, and COX-3 and the future treatment of chronic inflammatory disease. Lancet 2000;355:646-648.
2 Smith, et al., Pharmacological Analysis of Cyclo-oxygenase-1 in Inflammation. Proc. Natl. Acad. Sci. USA, Pharmacology 1998;95:13313-13318.
3 Jones CJ and Budsberg SC. Physiologic characteristics and clinical importance of the cyclooxygenase isoforms in dogs and cats. JAVMA 2000;217(5):721-729.
4 Zhang, et al., Inhibition of Cyco-oxygenase-2 Rapidly Reverses Inflammatory Hyperalgesia and Prostaglandin E2 Production, JPET 1997;283:1069-1075.
5 Jones and Budsberg, pp. 721-729.
6 Zhang, et al., pp. 1069-1075.
7 Chandrasekharan NV, Dai H, et al. COX-3, a cyclooxygenase-1 variant inhibited by acetaminophen and other analgesic/antipyretic drugs: Cloning, structure and expression. Proc. Natl. Acad. Sci. USA, 2002;99(21):13926-13931.
8 Data on file.
Made in Canada
Marketed by: Merial LLC, Duluth, GA 30096-4640, USA.
1-877-217-3543
U.S. Patent Nos. 5,981,576; 6,541,646; and 6,677,373
NADA 141-230, Approved by FDA
©2009, 2010 Merial. All Rights Reserved.
®PREVICOX is a registered trademark of Merial.
1050-1727-07
Rev. 03-2009
|
|
|
Product |
|
|
57 mg |
3 Chewable Tablets |
|
1039-2665-01 Rev. 09-2009 |
|
57 mg |
10 Chewable Tablets |
279160 |
036 834092 Rev. 08-2010 |
|
57 mg |
30 Chewable Tablets |
279120 |
036 834093 Rev. 08-2010 |
|
57 mg |
60 Chewable Tablets |
279150 |
036 834115 Rev. 09-2009 |
|
227 mg |
3 Chewable Tablets |
|
1039-2667-01 Rev. 09-2009 |
|
227 mg |
10 Chewable Tablets |
279170 |
036 902274 Rev. 12-2010 |
|
227 mg |
30 Chewable Tablets |
279130 |
036 834095 Rev. 08-2010 |
|
227 mg |
60 Chewable Tablets |
279140 |
036 834116 Rev. 09-2009 |
CPN: 11111093
3239 SATELLITE BLVD., DULUTH, GA, 30096
| Telephone: | 800-325-9167 | |
| Fax: | 816-236-2717 | |
| Website: | www.bevaccinesmart.com | |
| www.bi-vetmedica.com | ||
| www.haveweseenyourcatlately.com | ||
| www.metacam.com | ||
| www.mycathasdiabetes.com | ||
| www.prrsresearch.com | ||
| www.prozinc.us | ||
| www.vetera-vaccines.com | ||
| www.vetmedin-us.com | ||
| www.yourdogsheart.com | ||
| Email: | info@productionvalues.us |
 |
Every effort has been made to ensure the accuracy of the PREVICOX Chewable Tablets information published above. However, it remains the responsibility of the readers to familiarize themselves with the product information contained on the US product label or package insert. |

Copyright © 2024 Animalytix LLC. Updated: 2024-02-27
