Metacam 40 mg/mL Solution for Injection (Canada)
This treatment applies to the following species: Company: Boehringer Ingelheim Animal Health
Company: Boehringer Ingelheim Animal Health
Meloxicam
Veterinary Use Only
Sterile
DIN 02529890
Each mL contains 40 mg meloxicam and 150 mg ethanol, as preservative.
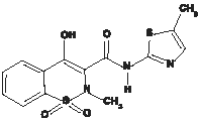
Meloxicam has a chemical formula of C14H13N3O4S2. The molecular weight is 351.4 g/mol, and its chemical structure is:
Therapeutic Classification: Nonsteroidal anti-inflammatory drug (NSAID).
Metacam 40 mg/mL Solution for Injection Indications
Cattle: As an aid in improving appetite and weight gains when administered at the onset of diarrhoea, in combination with oral rehydration therapy, in calves over one week of age.
For relief of pain following de-budding of horn buds in calves less than 3 months of age.
For the symptomatic treatment of inflammation and pain associated with acute clinical mastitis.
For the reduction of pain associated with abdominal surgery such as caesarean section.
Dosage and Administration
Cattle: Single subcutaneous or intravenous injection of 0.5 mg meloxicam/kg body weight (1.25 mL/100 kg). For reduction in pain associated with abdominal surgery, administer 10 to 20 minutes before the painful procedure.
Contraindications
Do not use in animals suffering from gastrointestinal disorders such as irritation and hemorrhage, impaired hepatic, cardiac or renal function and hemorrhagic disorders, or where there is individual hypersensitivity to the product.
Do not administer concurrently with steroidal, other nonsteroidal anti-inflammatory drugs or with anti-coagulant agents.
Concomitant use of NSAIDs with aminoglycoside antimicrobials in very young animals may result in renal toxicity.
Cautions: Do not use in bulls intended for breeding.
Avoid use in very severely dehydrated, hypovolaemic or hypotensive animals which require parenteral rehydration, as there may be a potential risk of increased renal toxicity.
Use of anti-inflammatories in very young or debilitated animals may involve additional risk.
Available data suggest that meloxicam has no harmful effects in the second and third trimester of pregnancy. However, the potential effects of meloxicam on imminent parturition have not been evaluated.
Treatment of cows with meloxicam before abdominal surgery reduces post-operative pain. An anti-inflammatory alone will not provide adequate pain relief during the surgical procedure. To obtain adequate pain relief during surgery, co-medication with an appropriate analgesic is recommended.
Warnings
- Treated cattle must not be slaughtered for use in food for at least 20 days after the latest treatment with this drug.
- Milk taken from treated cows during treatment and within 96 hours after the latest treatment with this drug must not be used in food.
- Do not use in calves to be processed for veal as a withdrawal period has not been established for pre-ruminating calves.
- People with known hypersensitivity to nonsteroidal anti-inflammatory drugs (NSAIDs) should not handle this product.
- Caution should be taken to avoid accidental self-injection, ingestion and contact with eyes.
- This product can cause eye irritation. In case of contact with the eyes, immediately rinse thoroughly with water.
- Keep out of reach of children.
Adverse Reactions
Transient, slight injection site swellings may occasionally be seen following subcutaneous injection and intravenous injection. In very rare cases, anaphylactoid reactions may occur and should be treated symptomatically.Pharmacology
Meloxicam is a nonsteroidal anti-inflammatory drug (NSAID) of the oxicam class which acts by inhibition of prostaglandin synthesis, thereby exerting anti-inflammatory, analgesic and anti-pyretic properties. Meloxicam also has anti-endotoxic properties because it has been shown to inhibit production of thromboxane B2 induced by E. coli endotoxin administration in calves.
Toxicology: The acute oral toxicity (LD50) of meloxicam assessed in rats, mini-pigs, mice and rabbits was >80 mg/kg, 1600 mg/kg, 470 mg/kg and 320 mg/kg, respectively. Repeated dose toxicity studies in rats, mice and mini-pigs demonstrated that the primary target organs for toxicity were the gastrointestinal tract (pyloric, duodenal and small intestine ulceration) and kidneys (scarring, necrosis and pyelonephritis).
Storage
Store between 15°C and 30°C. Keep from freezing.Presentation: Metacam® 40 mg/mL Solution for Injection is available in 100 mL multi-dose vials.
Metacam® is a registered trademark of Boehringer Ingelheim Vetmedica GmbH, used under license.
Boehringer Ingelheim Animal Health Canada Inc., 5180 South Service Road, Burlington ON L7L 5H4
Revised: 07-2022
CPN: 1182184.0
5180 SOUTH SERVICE ROAD, BURLINGTON, ON, L7L 5H4
| Customer Care No.: | 1-800-567-1885 | |
| Technical Services No.: | 1-877-565-5501 | |
| Website: | www.boehringer-ingelheim.ca |
 |
THIS SERVICE AND DATA ARE PROVIDED "AS IS". Animalytix assumes no liability, and each user assumes full risk, responsibility, and liability, related to its use of the Animalytix service and data. See the Terms of Use for further details. |

Copyright © 2024 Animalytix LLC. Updated: 2024-02-27
