advantage multi 100 (Canada)
This treatment applies to the following species: Company: Elanco
Company: Elanco
Pr advantage multi™ 10
(imidacloprid and moxidectin topical solution)
Topical parasiticide for dogs and puppies 7 weeks of age and older, weighing up to 4.5 kg.
DIN 02263556
Pr advantage multi™ 20
(imidacloprid and moxidectin topical solution)
Topical parasiticide for dogs and puppies 7 weeks of age and older, weighing 4.6 to 9 kg.
DIN 02263548
Pr advantage multi™ 55
(imidacloprid and moxidectin topical solution)
Topical parasiticide for dogs weighing 9.1 to 25 kg.
DIN 02263521
Pr advantage multi™ 100
(imidacloprid and moxidectin topical solution)
Topical parasiticide for dogs weighing 25.1 to 45 kg.
DIN 02263564
VETERINARY USE ONLY
Description
advantage multi (imidacloprid and moxidectin topical solution) is a clear to slightly opalescent liquid, yellow to brownish ready-to-use solution packaged in single dose applicator tubes. Imidacloprid is a chloronicotinyl nitroguanidine insecticide. The chemical composition of imidacloprid is 1-{(6-Chloro-3-pyridinyl)methyl}-N-nitro-2-imidazolidinimine. Moxidectin is a semisynthetic macrocyclic lactone endectocide derived from the actinomycete Streptomycetes cyaneogriseus noncyanogenus.
advantage multi 100 Indications
advantage multi is indicated for the prevention of cardiovascular dirofilariasis (heartworm disease) caused by Dirofilaria immitis and angiostrongylosis (French heartworm) caused by L4 larvae and immature adults of Angiostrongylus vasorum, for the treatment and control of the adult stage of fox lungworm (Crenosoma vulpis), the treatment and control of parasitic infestations caused by the adult stage of the common flea (Ctenocephalides felis), ear mites (Otodectes cynotis), sarcoptic mange mites (Sarcoptes scabiei var. canis), and as an aid in the treatment and control of generalized demodectic mange (Demodex canis).
advantage multi is also indicated for the treatment and control of parasitic infections caused by the developing L4, the sexually immature adult and the adult stages of hookworms (Ancylostoma caninum and Uncinaria stenocephala), the adult stage of ascarids (Toxocara canis and Toxascaris leonina), and as an aid in the treatment and control of whipworm infections caused by the adult form of Trichuris vulpis.
For the Prevention of:
|
Parasite |
|
|
Heartworm Species |
Dirofilaria immitis Angiostrongylus vasorum |
For the Treatment and Control of:
|
Parasite |
Parasitic Stage |
|||
|
Adult |
Immature Adult |
Fourth Stage Larvae |
||
|
Fleas |
Ctenocephalides felis |
X |
|
|
|
Ear Mites |
Otodectes cynotis |
X |
|
|
|
Sarcoptic Mange Mites |
Sarcoptes scabiei var. canis |
X |
|
|
|
Lungworm |
Crenosoma vulpis |
X |
|
|
|
Hookworm Species |
Ancylostoma caninum |
X |
X |
X |
|
Uncinaria stenocephala |
X |
X |
X |
|
|
Roundworm Species |
Toxocara canis |
X |
|
|
|
Toxascaris leonina |
X |
|
|
|
As an Aid in the Treatment of:
|
Parasite |
Parasitic Stage |
|||
|
Adult |
Immature Adult |
Fourth Stage Larvae |
||
|
Demodectic Mange Mites |
Demodex canis |
X |
|
|
|
Whipworm |
Trichuris vulpis |
X |
|
|
DOSAGE: Apply to the skin at the recommended minimum dose of 8.8 mg of imidacloprid and 2.2 mg of moxidectin per kg of body weight, once a month.
ADMINISTRATION:
1. Remove one unit dose tube from the package.
As specified in the following table, administer the entire contents of the advantage multi applicator tube that correctly corresponds with the body weight of the dog.
|
Dog weight (kg) |
advantage multi |
Volume (mL) |
mg Imidacloprid |
mg Moxidectin |
|
Up to 4.5 |
advantage multi 10 |
0.4 |
40 |
10 |
|
4.6 to 9 |
advantage multi 20 |
1.0 |
100 |
25 |
|
9.1 to 25 |
advantage multi 55 |
2.5 |
250 |
62.5 |
|
25.1 to 45* |
advantage multi 100 |
4.0 |
400 |
100 |
* Dogs over 45 kg should be treated with the appropriate combination of advantage multi tubes.
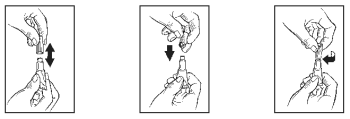
2. While holding the tube in an upright position, remove the cap from the tube.
3. Turn the cap over and place the other end of cap onto the tip of the tube.
4. Twist the cap to break the seal and then remove cap from the tube.
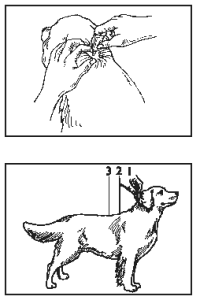
The dog should be standing for application. Part the hair on the dog’s back until the skin is visible. Place the tip of the tube on the skin and squeeze the entire contents directly on the exposed skin at one spot between the shoulder blades. Apply slowly to allow absorption. Do not apply an amount of solution at any one location that could run off the side of the dog. Do not get this product in your pet’s mouth or eyes. Stiff hair, a damp appearance of the hair, or a slight powdery residue may be observed at the treatment site on some animals. Until advantage multi dries, keep pet off furniture and away from hardwood floors.
Exposure to water, as may occur with swimming or rain fall, beginning 60 minutes after treatment, or shampooing or bathing 90 minutes after treatment, does not reduce the effectiveness of advantage multi in the prevention of heartworm disease.
Treatment and Control of Flea Infestations:
For the treatment and control of flea infestations, advantage multi should be administered at one-month intervals throughout the flea season. If the dog is infested with fleas when the first dose of advantage multi is administered, adult fleas on the dog will be killed. However, re-infestation from the emergence of pre-existing pupae in the environment may continue to occur for six weeks or longer after treatment is initiated.
Dogs treated with advantage multi including those with pre-existing flea allergy dermatitis have shown clinical improvement as a direct result of elimination of fleas from the dog.
Heartworm Disease Prevention:
Dirofilaria immitis:
For the prevention of cardiovascular dirofilariasis (heartworm disease) caused by Dirofilaria immitis:
advantage multi must be administered at one-month intervals during the time of the year when mosquitoes are present. Administration of advantage multi should start one month after the first expected exposure to mosquitoes and should continue at monthly intervals until one month after the last exposure to mosquitoes. When replacing another monthly heartworm preventative product in a heartworm prevention program during heartworm season, the first treatment with advantage multi must be given within one month of the last dose of the previous monthly administered medication.
advantage multi may be safely administered to heartworm infected dogs. However, it is recommended, in accordance with good veterinary practices, that all dogs > 6 months of age should be tested for patent heartworm infections before beginning prophylactic medication for the first time. After the first season on medication, all treated dogs should be retested at the start of the next season to certify treatment compliance before prescribing further medication. Thereafter, the determination of the frequency of testing should be left at the discretion of the Veterinarian.
Angiostrongylus vasorum:
For the prevention of angiostrongylosis (French heartworm) caused by Angiostrongylus vasorum:
advantage multi must be administered at one-month intervals during the time of the year when terrestrial gastropods (i.e. snails and slugs) and frogs are present. Administration of advantage multi should start one month after the onset of frog and terrestrial gastropod activity and continue at monthly intervals until one month after the end of activity.
Dogs acquire infection by the ingestion of terrestrial gastropods or frogs containing infective third-stage larvae (L3) of A. vasorum. While it is unknown if L3 larvae survive over winter in frogs and terrestrial gastropods, the transmission season for A. vasorum is presumed to equal the period when they would be active and therefore accessible for dogs to ingest.
Treatment and Control of Intestinal Nematode Infections:
advantage multi will remove the adult, the sexually immature adult and the developing L4 stages of intestinal hookworms (Ancylostoma caninum and Uncinaria stenocephala), the adult stage of ascarids (Toxocara canis and Toxascaris leonina), and aid in the treatment and control of the adult stage of whipworms (Trichuris vulpis). Monthly treatment with advantage multi will control the development of these intestinal nematode infections.
Treatment and Control of Fox Lungworm (Crenosoma vulpis):
For the treatment and control of the adult stage of the fox lungworm (Crenosoma vulpis), administer a single dose monthly.
Treatment and Control of Mite Infestations:
For the treatment and control of ear mite infestation (Otodectes cynotis), administer a single dose. Administer a second monthly dose if the dog remains mite positive at 4 weeks or in the case of re-infestation.
For the treatment and control of sarcoptic mange (Sarcoptes scabiei var. canis), administer monthly for two applications.
As an aid in the treatment and control of generalized demodectic mange (Demodex canis), administer monthly for four applications. As demodicosis is a multi-factorial disease, where possible, it is advisable to also treat any underlying disease appropriately.
Monthly use of advantage multi will treat any subsequent mite infestations.
Contraindications
Do not administer orally. See the Animal Safety Section.
CAUTIONS:
Use with caution in sick, debilitated, or underweight animals.
Use with caution in dogs with MDR1 mutation. Dogs with this mutation are more sensitive to avermectins and may develop clinical signs of severe avermectin toxicity (depression, salivation, dilated pupils, incoordination, generalized muscle tremors, coma, death) if they ingest this product. The most common breeds with this mutation include collies and collie crosses. See the Animal Safety section.
The safe use of advantage multi in dogs used for breeding purposes, during pregnancy, or in lactating bitches has not been evaluated.
Warnings
Keep this product, and all drugs, out of the reach of children.
May be irritating to skin and eyes. If contact with eyes occurs, flush eyes copiously with water. If eye irritation persists, contact a physician. Wash hands after use. If contact with skin occurs, wash skin immediately with soap and water. If ingested, contact a physician immediately.
Adverse Reactions
Use of the product may result in transient pruritus in dogs. Rarely, greasy fur, erythema and vomiting can occur. These signs disappear without further treatment. The product may, in rare cases, cause local hypersensitivity reactions. If the animal licks the application site after treatment, transient, self-limiting neurological signs such as ataxía, generalized tremors, ocular signs (dilated pupils, little pupillary reflex, nystagmus), abnormal respiration, salivation and vomiting may occur infrequently.
The product tastes bitter. Salivation may occasionally occur if the animal licks the application site immediately after treatment. This is not a sign of intoxication and disappears within some minutes without treatment. Correct application will minimize licking of the application site. The product may in very rare cases cause at the application site a sensation resulting in transient behavioural changes such as lethargy, agitation, and inappetence. In case of accidental oral uptake, symptomatic treatment should be performed by a Veterinarian. There is no known specific antidote. The use of activated charcoal may be beneficial. To report suspected adverse drug events or for technical assistance, contact Elanco Canada Limited at 1-800-265-5475.
Post-market experience:
Although all adverse reactions are not reported, the following adverse reaction information is based on voluntary post-approval drug experience reporting. It is generally recognized that this method of reporting results in significant under-reporting of adverse drug reactions. It should be noted that suspected adverse drug reactions listed here reflect reporting and not causality. The categories of adverse reactions are listed in decreasing order of frequency by body system:
Systemic disorders: lethargy, inappetence, lack of efficacy
Behavioural disorders: agitation, vocalization, disorientation, aggression, anxiety, inappropriate defecation/urination, excessive appetite
Digestive tract disorders: salivation, vomiting, diarrhea
Application site disorders: pruritus, erythema, alopecia, dermatitis, lesion at the application site
Neurological disorders: ataxia, tremor, twitching, mydriasis, paresis, nystagmus, hyperesthesia
Skin and appendages disorders: pruritus, erythema, alopecia, dermatitis, lesion, pyoderma
Respiratory tract disorders: panting, cough, tachypnea, dyspnea
Immune system disorders: urticaria, allergic edema
ANIMAL SAFETY:
Clinical Field Trials: advantage multi was evaluated in a controlled, double-blind, multi-site clinical field trial. Owners of the 196 dogs participating in the study made a total of 1,764 observations at designated post-treatment intervals. Veterinarians classified the majority of these observations (1,674/94.9%) as normal.
In dogs treated with advantage multi, the observations classified as abnormal included application site residue, inappetence, lethargy, medicinal odor/ breath and pruritus.
In clinical field studies, advantage multi was used safely in dogs receiving other frequently used veterinary products such as vaccines, antiparasitics, antibiotics, steroids, pesticides and shampoo.
Safety Studies in Puppies: advantage multi was applied topically at 1, 3, and 5 times the recommended dose to puppies between 6 and 7 weeks old once every 2 weeks for a period of 12 weeks. A control group of puppies was treated with topical applications of mineral oil at 5 times the recommended volume of advantage multi over the same time intervals.
Clinical signs seen in some animals from all groups (treatment and control) were occasional soft stools, loose stools, diarrhea, mucous in the stool, and/or blood in the stool. Additionally, ocular discharge was observed in a few animals in each of the study groups. All of the above are clinical signs commonly seen in puppies within this age range. A rough hair coat was observed in some puppies from all groups.
In the group receiving the recommended dose, 1 animal was observed with otitis externa and lesions on the muzzle for 6 days and 2 animals were observed with transient white residue on the hair coat at the site of product application.
In the group receiving 3 times the recommended dose, transient vomiting was seen in 2 animals and 5 animals had transient white residue on the hair coat at the product application site.
At 5 times the recommended dose, transient vomiting was seen in 1 animal and 4 animals had transient white residue on the hair coat at the product application site. One animal in the group experienced transient heavy breathing on study day 13. Raspy and difficult breathing continued for 4 hours on study day 14.
Dermal Dose Tolerance Study: advantage multi was administered once topically to 7-8 month old dogs at 10 times the recommended dosage. There were no adverse clinical signs exhibited during the study. In addition, clinical chemistry or hematology values were not adversely affected by administration of the test article.
Safety Study in Heartworm-Positive Dogs: advantage multi was administered topically at 1 and 5 times the recommended dosage to dogs with adult heartworm infections and circulating microfilariae. A control group of heartworm-positive dogs received topical applications of mineral oil at 5 times the recommended volume of advantage multi. Each animal received 1 treatment every 14 days for a total of 3 treatments. In the control group, 1 dog had loose stools and 1 dog was observed with diarrhea during the observation period. In the group receiving the recommended dose, 1 dog had ocular discharge for 1 day. At 5 times the recommended dose, 1 dog was observed vomiting 3 hours after the second treatment. No additional hypersensitivity reactions have been observed in heartworm infected dogs when advantage multi was administered at 5 times the labeled dose.
Safety Study in Dogs with Natural Heartworm Infections: advantage multi was administered topically at 5 times the recommended dose to naturally infected heartworm positive dogs. A group of control dogs received a topical application of mineral oil at 5 times the recommended volume. Each animal received 1 treatment every 14 days for a total of 3 treatments. In the dogs receiving 5 times the recommended dose, 1 dog was observed to have a mild skin reaction on the day after each application and 1 dog vomited undigested food 1 day after the first treatment. No other signs were noted.
Oral Safety Study in Dogs: advantage multi was administered once orally at the recommended topical dose to address accidental ingestion. Twelve dogs received advantage multi and 12 dogs received water as a control. Of the dogs receiving advantage multi, 6 dogs vomited within 1 hour of receiving the test article and 1 dog vomited up to 18 hours post-dosing. One dog exhibited abnormal neurological signs (circling, ataxia, generalized muscle tremors, and dilated pupils with a slow pupillary light response) for 4 hours post-dosing. This dog was normal at 24 hours post-dosing and throughout the remainder of the study.
Dermal Safety Study in Ivermectin-Sensitive Collies: advantage multi was administered dermally at 3 and 5 times the recommended dosage to Collies which had been pre-screened for ivermectin sensitivity. No clinical abnormalities were observed.
Oral Safety Study in Ivermectin-Sensitive Collies: advantage multi was administered orally to 5 pre-screened ivermectin-sensitive Collies at less than the recommended dose. Four of the dogs experienced signs indicative of ivermectin induced toxicity.
Storage
Store at temperatures between 4° and 30°C, avoiding excess heat or cold.How Supplied
|
Applicator Tube Size |
Applications Per Package |
|
0.4 mL |
6 X 0.4 mL tubes |
|
1.0 mL |
6 X 1.0 mL tubes |
|
2.5 mL |
6 X 2.5 mL tubes |
|
4.0 mL |
6 X 4.0 mL tubes |
Elanco Canada Limited, 1919 Minnesota Court, Suite 401, Mississauga, Ontario L5N 0C9
advantage multi, Elanco and the diagonal bar logo are trademarks of Elanco or its affiliates.
© 2022 Elanco or its affiliates.
31May2022
CPN: 1231194.1
1919 MINNESOTA COURT, SUITE 401, MISSISSAUGA, ON, L5N 0C9
| Customer Service: | 800-265-5475 | |
| Fax: | 519-821-7831 | |
| Website: | www.elanco.ca | |
| Email: | elancocanadacustomerservice@elancoah.com |
 |
THIS SERVICE AND DATA ARE PROVIDED "AS IS". Animalytix assumes no liability, and each user assumes full risk, responsibility, and liability, related to its use of the Animalytix service and data. See the Terms of Use for further details. |

Copyright © 2024 Animalytix LLC. Updated: 2024-02-27
